
Atlas combines an intoxicating blend of high-tech science, engineering, refinement in 3D measurements.
FEATURES AND BENEFITS
- Available both as a 3D imaging extension for various types of existing microscopes and as an individual system as well
- Simultaneous read out of neural activity on both the somatic and dendritic scales
- Increased measurement speed and signal-to-noise ratio by several orders of magnitude
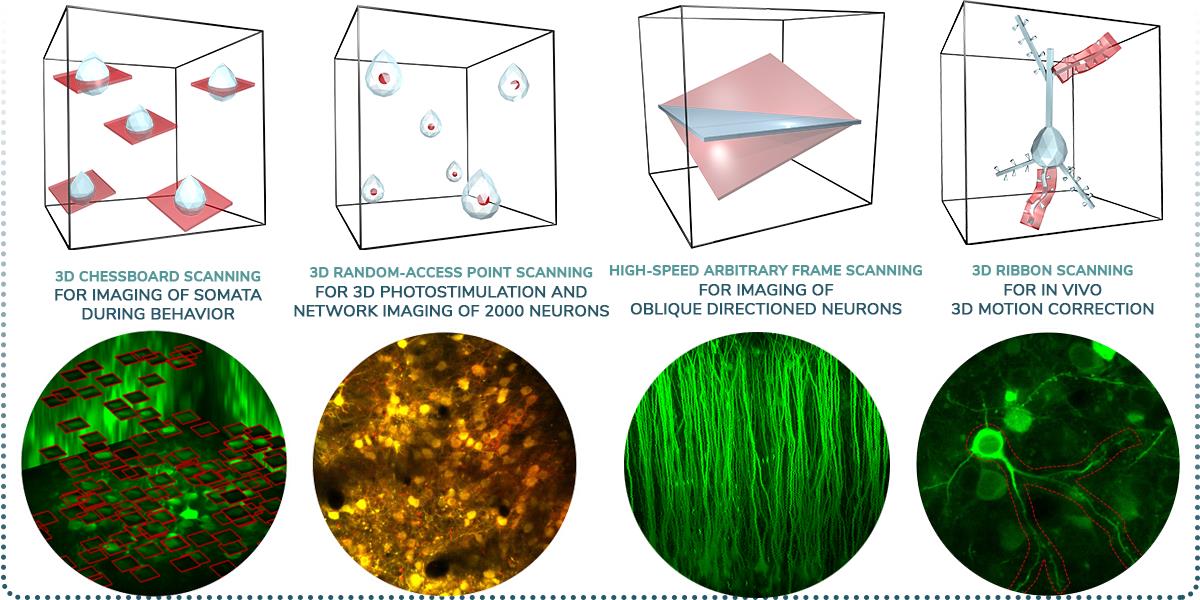
- 3D Network imaging of over 2000 soma distributed in 3D
- Imaging of dendritic branches through layers
- Simultaneous imaging of over thousands of spines
- Unique, flexible scanning methods supporting in vivo and in vitro measurements
- In vivo real-time 3D motion correction
- High-speed 3D random-access ROI scanning: scanning speed up to 100 kHz to any points in 3D
- High-speed raster scanning: 40 fps at 510×510, and even 3000 fps at reduced FOV
- Improved excitation efficiency for large 3D scanning volume with GECIs
- Automatic wavelength tuning between 750 – 1050 nm
- Functional imaging correlated with electrophysiological data
- Calcium imaging during behavior
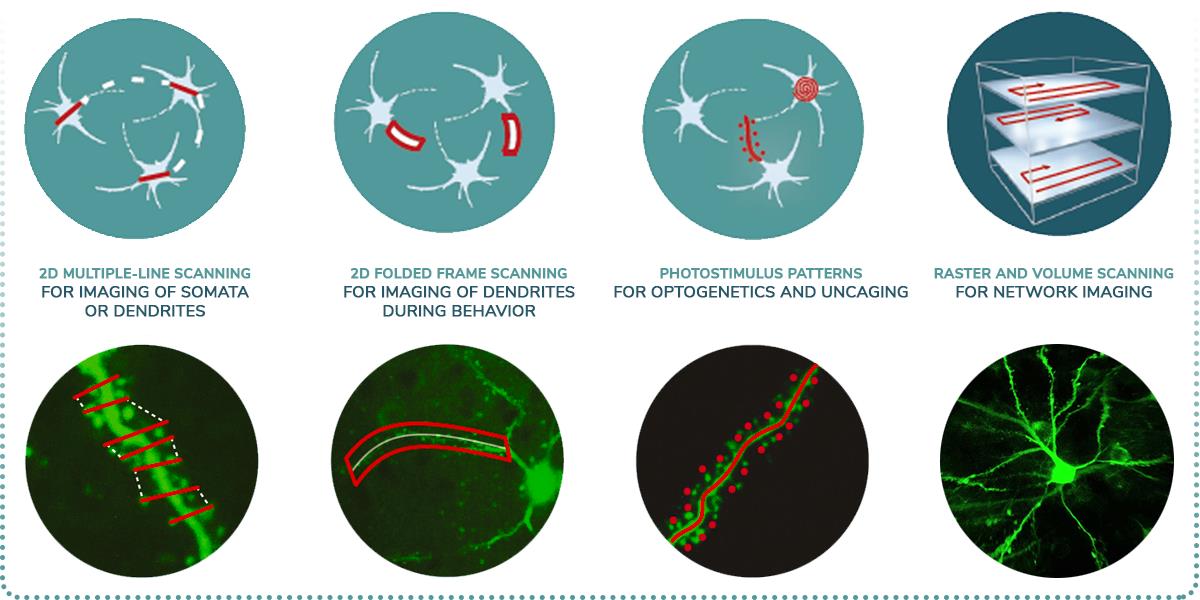
- 3D random-access photo stimulation for optogenetics
Femto
Smart
The FEMTOSmart series is the next step in classical two-photon microscopy at Femtonics, fully customizable two-photon microscopes.
FEATURES AND BENEFITS
- Elevated, column-based body
- XYZ positioning
- Tilting objective
- High level of modularity
- Galvanometric, resonant, or dual scanning possibilities
- Galvo Scanner, focusing on regions-of-interest
- Resonant Scanner, fast frame and volume scanning
- Dual Scanner, advantages of Galvo and Resonant microscopes combined
- Travelling detector system
- For in vivo studies
- High number of optional modules
- Patented imaging technologies
- Flexible scanning methods
- Maximal photon collection
FOCUSPINNER
for behaving animals
/Compatible with FEMTO3D Atlas/
Technology Overview
Enhance your in vivo measurements effortlessly with the Femtonics FocusPinner, which offers Real-Time Motion Correction along X,Y and Z (axial) direction, effectively eliminating motion artifacts.
Neuroscience relies more heavily on in vivo measurements of neuronal activity to study cognition, memory, CNS diseases, behaviour, neurochemistry, and many other aspects of the field. One of the bottlenecks of in vivo functional measurements is motion artifacts caused by the movement of the animal, respiration, circulation, or any of the numerous other sources. Here we offer a real-time solution for in vivo motion correction which eliminates artifacts caused by heartbeat, breathing or physical activity at over 100 kHz speed allowing calcium and voltage imaging from spines, dendrites, axons and somata with high resolution.
Benefits
- Acousto-optic based: By leveraging the capabilities of 3D random-access line scanning, our system enables seamless motion correction along each axis. It offers a significant advantage over the currently available XY plane cross-correlation-based solutions by providing feasible Z directional (axial) correction.
- Useful at in-vivo measurements: motion artifacts caused by the movement of the animal, respiration, circulation can be efficiently eliminated (average residual motion < 10%).
- Real-time: During the measurement, the focal point is on-line relocated based on the estimated motion of the reference point. Compared to quasi-real time solutions the reaction time is one-tenth.
- Reaction time: The cycle of repeated motion correction is less than 100 µs, meaning that the largest residual motion amplitude is the one collected during this short time-window.
- Scanning modes: It is compatible with all scanning modes (e.g. raster, chessboard, multicube, longitudinal and transverse ribbon, snake, Z-Stack) of the FEMTO3D Atlas system.
FemtoStage
for high-precision experiments
/Compatible with most microscope systems/
Technology Overview
The FemtoStage is a high-quality motorized XY stage, with a flexible sample holder. It can move the sample in your microscope with high precision, more than enough for any experimental needs. Multiple configurations are available, and it can be further customized for the specific needs of the user. It is suitable for both in vivo and in vitro experiments, with many neurobiological applications. This includes, but is not limited to patch-clamping, complex behavior experiments, network imaging, voltage imaging, and reward learning experiments.
Features
- Size: 600 × 510 × 73mm (without legs)
- Travel range: ± 35 mm (XY, motorized), 45 mm (Z, manual)
- Load capacity: 6kg
- Minimum step size: 50 nm
DICHRO EXTENSION
for flexible photostimulation
/Compatible with FEMTO3D Atlas/
Technology Overview
The Dichro option of the ATLAS product line allows you to use two independent laser sources with two different wavelengths in the same ATLAS scanhead. Since the acousto-optic deflection depends on the wavelength of light, different driving of the acousto-optic crystals selects only one of the input beams to pass through the scanhead. As a result, the user is able to select for each approximately 30 us long AO cycle, which input beam to focus within the 3D space of the sample. In-depth protocol editor and MultiPulse generator windows let you control imaging and photo-stimulation down to the finest details.
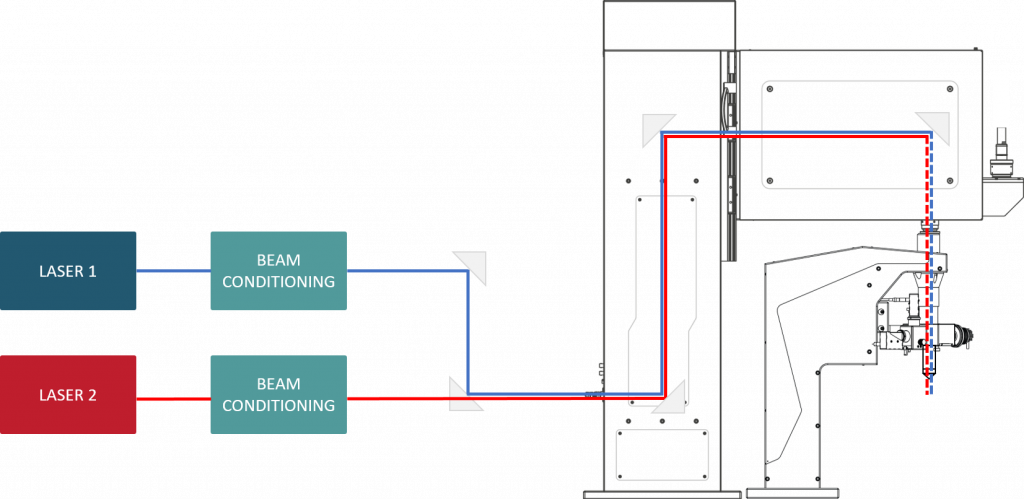
Features
- Separate automatic beam stabilization for both laser beams
- 1st laser: 750-970 nm, 2nd laser: 1030-1050 nm (standard coupling dichroic mirror)
- Compatible with tunable and fiber laser sources
- Switching between wavelengths during the dead time of the AODs (<15 us)
- Complex software architecture for controlling spatiotemporal pattern of scanning
- Up to 100 kHz in vivo
Benefits
- Optogenetics and uncaging experiments
- Use two differently tuned optogenetic labels at the same time, without compromise
- Predictable irradiation energy over the variation of photo-stimulation pattern (unlike in case of holographic illumination)
- Quasi-instantaneous and inertia-free switching between imaging and photo-stimulation
- Stimulation of 40 cells within 16.8 ms
MULTIPLE BEAM PATH
for uncaging and optogenetics
/Compatible with FEMTOSmart/
Technology Overview
The optomechanical design of the light path enables us to direct more beams into the microscope, utilizing the same light path. We offer secondary, fine-tuned laser sources for a wide range of biophotonics applications. See more: Optogenetics, Uncaging.

Features
- Additional IR or CW laser(s) coupled to the existing light path
- Lightpath can be optimized for three wavelength ranges (450-1100 or 700-1100 or 900-1300 nm)
- Full optical engineering
- Software controlled operation
Benefits
- Photostimulation with IR or visible light
- Flexible stimulation patterns supporting stimulation of selected regions
- Two-photon uncaging studies
- Visible light optogenetics:
- ChR2 activation with 473 nm laser
- NpHR activation with 561 nm laser
IN VITRO EXTENSION KIT
for cultured cells or acute brain slices
/Compatible with FEMTO3D Atlas and FEMTOSmart/
Technology Overview
Imaging acute brain slices or cultured cells and tissues enables the user to study cells in a controlled environment, outside of a living organism. Gradient contrast illumination eases camera guided patch-clamping while transmitted fluorescence detectors enhance signal collection and SNR.
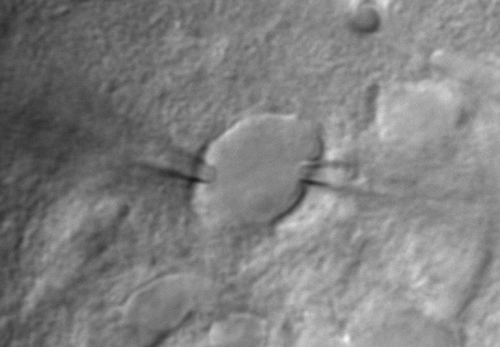
Features:
- Transmission Köhler illumination with a high-power IR LED (850nm)
- High NA condenser (dry or oil immersion options)
- Visible light detection (Fluorescence, SHG, THG, CARS, etc): efficient, large aperture light collecting optics with visible detection ports between 400-690 nm, a maximum of 2 visible light detectors (options: GaAsP, gated GaAsP, multialkali PMT or hybrid detectors for FLIM), and a manually exchangeable filter cube
- Transmission infrared detector with a TIR detection port between 720-1070 nm (options: biased silicon photodiode or multialkali PMT)
Benefits:
- An easily replaceable protective window with waterproof sealing safeguards the sensitive internal optics from leaking water and corrosive solutions during experiments
- Versatile mechanical solutions:
- Compatible with Femtonics microscopes (SMART with tiltable or normal objective arm, Platform), and with an LN stage
- The condenser, condenser holder or the whole module is removable prior to in vivo measurements, owing to the quick release adapter placed on the fix mount to the optical table
FLIM EXTENSION
for Fluorescence Lifetime Imaging
/Compatible with FEMTOSmart/
Technology Overview
Time correlated single photon counting and the derived fluorescence lifetime imaging measures the time delay between each emitted photon and the laser pulse eliciting it. This time is not affected by fluorophore concentration and excitation intensity fluctuations, however provides intimate information about molecular interactions and dynamics. See more: Applications – FLIM.
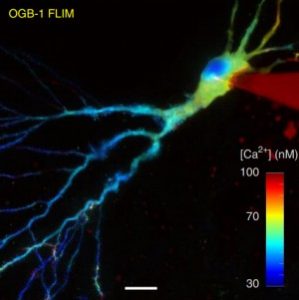
Features
- High efficiency hybrid GaAsP PMTs for photon counting
- No afterpulsing
- Up to two photon counting wavelength channels
- Non-descanned design for high photon collection rate
- Minimized optical path length by travelling detector system
- Sub-picosecond temporal resolution
- Parallel conventional integrating and photon counting modes
Benefits
- Absolute ion concentration measurement
- Characterization of bio-physical properties
Related Publications:
Fluorescence lifetime imaging reveals regulation of presynaptic Ca2+ by glutamate uptake and mGluRs, but not somatic voltage in cortical neurons. Olga Tyurikova, Kaiyu Zheng, Elizabeth Nicholson, Yulia Timofeeva, Alexey Semyanov, Kirill Volynski, Dmitri A. Rusakov, Journal of Neurochemistry (2020)
Local Resting Ca2+ Controls the Scale of Astroglial Ca2+ Signals. Claire M. King, Kirsten Bohmbach, Daniel Minge, Andrea Delekate, Kaiyu Zheng, James Reynolds, Cordula Rakers, Andre Zeug, Gabor C. Petzold, Dmitri A. Rusakov, Christian Henneberger, Cell Reports (2020)
Multiplex imaging relates quantal glutamate release to presynaptic Ca2+ homeostasis at multiple synapses in situ. Thomas P. Jensen, Kaiyu Zheng, Nicholas Cole, Jonathan S. Marvin, Loren L. Looger, Dmitri A. Rusakov, Nature Communications (2019)
Polymer microchamber arrays for geometry-controlled drug release: a functional study in human cells of neuronal phenotype. Olga Kopach, Kayiu Zheng, Olga A. Sindeeva, Meiyu Gai, Gleb B. Sukhorukov, Dmitri A. Rusakov, Biomaterials Science (2019)
Glutamate Imaging Reveals Multiple Sites of Stochastic Release in the CA3 Giant Mossy Fiber Boutons. Sylvain Rama, Thomas P. Jensen, Dmitri A. Rusakov, Front. Cell. Neurosci. (2019)
A genetically encoded fluorescent sensor for in vivo imaging of GABA. Jonathan S. Marvin, Yoshiteru Shimoda, Vincent Magloire, Marco Leite, Takashi Kawashima, Thomas P. Jensen, Ilya Kolb, Erika L. Knott, Ondrej Novak, Kaspar Podgorski, Nancy J. Leidenheimer, Dmitri A. Rusakov, Misha B. Ahrens, Dimitri M. Kullmann & Loren L. Looger, Nature Methods (2019)
Monitoring intracellular nanomolar calcium using fluorescence lifetime imaging. Kaiyu Zheng, Thomas P Jensen & Dmitri A Rusakov, Nature Protocols (2018)
Monitoring single-synapse glutamate release and presynaptic calcium concentration in organised brain tissue. Jensen TP, Zheng K, Tyurikova O, Reynolds JP, Rusakov DA, Cell Calcium (2017)
Time-Resolved Imaging Reveals Heterogeneous Landscapes of Nanomolar Ca2+ in Neurons and Astroglia. Kaiyu Zheng, Lucie Bard, James P. Reynolds, Claire King, Thomas P. Jensen, Alexander V. Gourine, Dmitri A. Rusakov, Neuron (2015)
EPIFLUORESCENT UNIT
for multicolor full-field illumination
/Compatible with FEMTOSmart/
Technology Overview
Wavelength-specific full-field excitation is performed by powerful LED light sources built in a rotating unit above the objective. Fluorescent imaging can be achieved with a wide variety of scientific cameras.
Features
- LEDs for customizable wavelengths (430, 450, 480, 530, 590 nm, others on special request)
- Wavelength-specific filters for excitation of narrow ranges
- Additional emission filters for blocking the reflected light and filtering the emission spectra
- Up to 5 sets of exchangeable filter cubes
- 100 µm penetration depth
- Detection by CMOS camera
- Software controlled operation
- Installed into the light path above the objective
- PMT protection during the stimulation by built-in gating system
Benefits
- Homogenous excitation of the entire FOV in 100 µm depth
- Excitation specified for GFP, YFP, RFP, mCherry, etc.
- Cleared emission for bright signals
BRIDGE STRUCTURE
for coarse Z movement
/Compatible with FEMTOSmart/
Technology Overview
The Bridge structure is a lifting apparatus for FemtoSmart which can replace the foot. It provides extreme freedom in positioning of the body and an increased space under the objective.
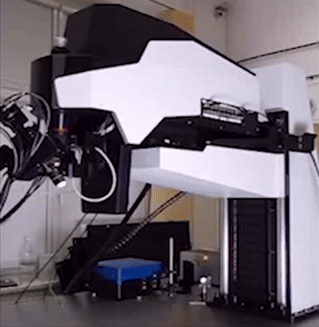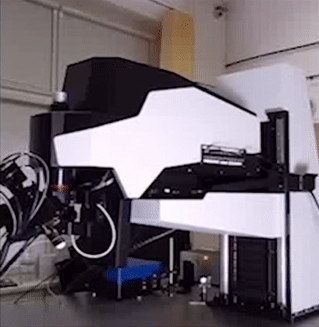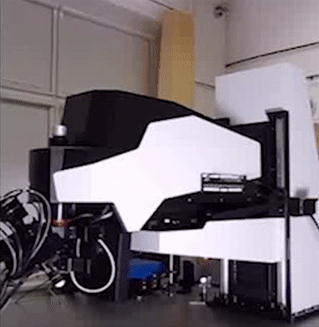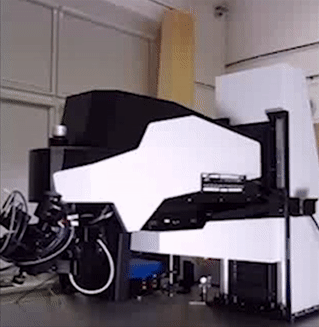
Features
- Moving range: 50 cm
- Coarse Z adjustment: 1 mm
- Distance between the foot and the objective: 880 mm
Benefits
- Very large space for the sample or accessories
- High-range mobility
- Locate your chronic imaging spot within the craniotomy
PIEZO OBJECTIVE POSITIONER KIT
for fast Z-stack and 3D imaging
/Compatible with FEMTOSmart/
Technology Overview
A Piezo objective positioner kit enables the microscope to change the focal point by mechanically moving the objective. With this module, the microscope is able to collect signals from different depths fast enough to resolve biological activity in 3D samples. We can equip most of our microscopes with two types of piezo: piezo specialized for large scanning volumes moves the objective in a 400 µm travel range with up to 30 Hz speed, and the faster one moves it in 100 µm volume with up to 100 Hz (the refresh rate depends on the scanning volume and the size of the objective).
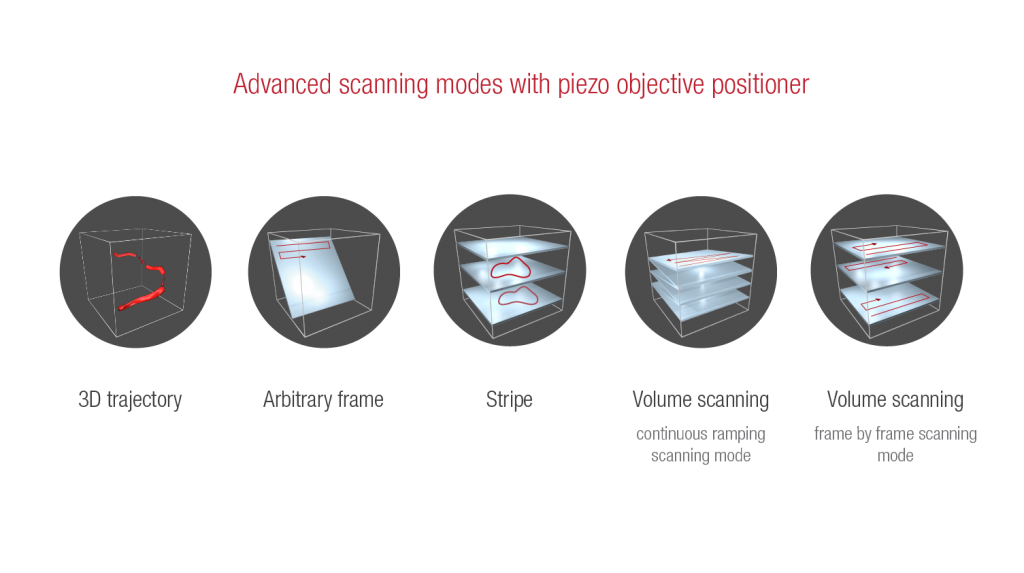
Features
- Positions objectives in the Z direction with nm resolution
- Traveling range 100 or 400 µm (depends of the piezo’s type)
- Up to 100 Hz frequency in resonant mode
- Millisecond step-and-settle
- Any types of objectives can be attached
- No influence on the optical path
Benefits
- Femtonics microscopes containing galvo scanner equipped with piezo objective positioner and RollerCoaster software module support 3D trajectory scanning
- 3D trajectory scanning allows collecting signals from dendrites arbored in the 3D tissue and to resolve biological activity thanks to the high scanning speed
- Femtonics microscopes containing resonant or galvo scanners are able to image a 3D volume scanning
- 3D volume scanning enables you to reveal the activity of the neural network or other cell groups
- Fine positioning along the tilted axis when using tilting objective module
MOTORIZED TILTING OBJECTIVE UNIT
for free rotation of the objective
/Compatible with FEMTOSmart/
Technology Overview
The motorized tilting module rotates the objective, giving a higher level of freedom to reach the sample from different angles. The module also includes a Piezo objective positioner, ensuring additional movement of the objective in the Z direction.
Applications
Novel surgical methods utilizing grin lenses or miniature prisms allow optical investigation of deep structures within the brain. Implementing these technologies is greatly enhanced by our motorized tilting objective:
- aligning optical axis of the embedded optical elements is facilitated by the handwheel operated multi-axis positioning of the grin lens/Smart microscope,
- store and reuse optimal angles and positions when experimenting multiple times with the same animal,
- utilize the units high-precision movements to fine adjust position and alignment without actually touching the assembly,
- suitable for imaging lateral brain regions, even in non-human primates.
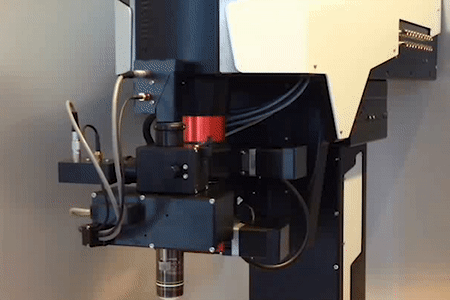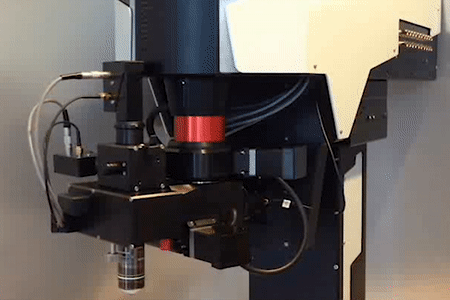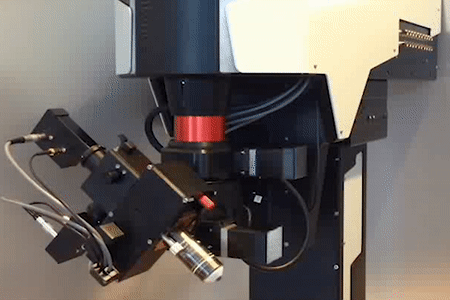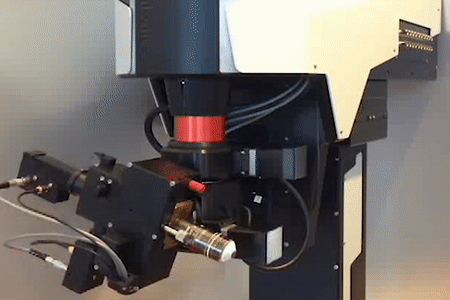
Features
- 180º rotation around the horizontal axis
- 100º rotation around the vertical axis
- Speed of rotation: 4º/sec
- Unidirectional repeatability: less than 0.02 mrad (~2 µm)
- Z movement with piezo objective positioner module: 400 µm
- Transmission over 80% (700-1100 or 900-1300 nm)
- Accepts both conventional and large back aperture objectives
- Green illumination module
- Maximum number of mounted detectors: 3
Benefits
- Flexible objective positioning with high precision
- Highly stable in all positions
- Fast z-stack acquisition also from tilted position
- Close coupled detectors
- Useful for in vivo experiments, intravital imaging, and deep brain imaging in rodents or even non-human primates
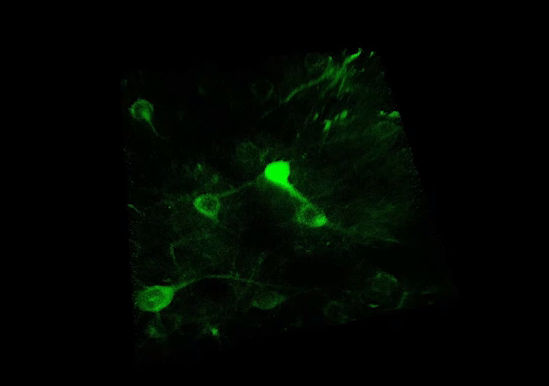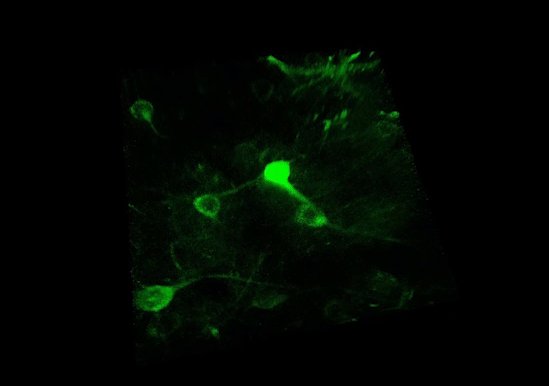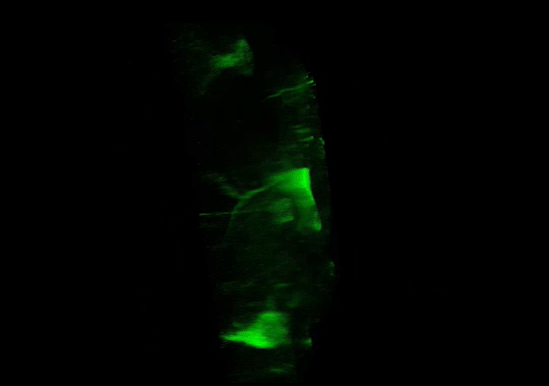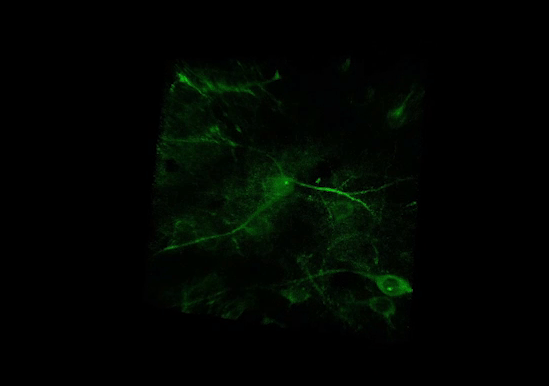
3P RANGE MICROSCOPY
for three-photon excitation
/Compatible with FEMTOSmart/
Technology Overview
Three-photon (3P) microscopy is a powerful technique that allows for the noninvasive structural and functional imaging of deep tissues with higher axial resolution compared to two-photon excitation. 3P excitation is performed at longer excitation wavelengths which scatter less in biological tissues. (See more at 3P imaging.) This extends the penetration depth, reduces out-of-focus excitation, and increases the SNR.
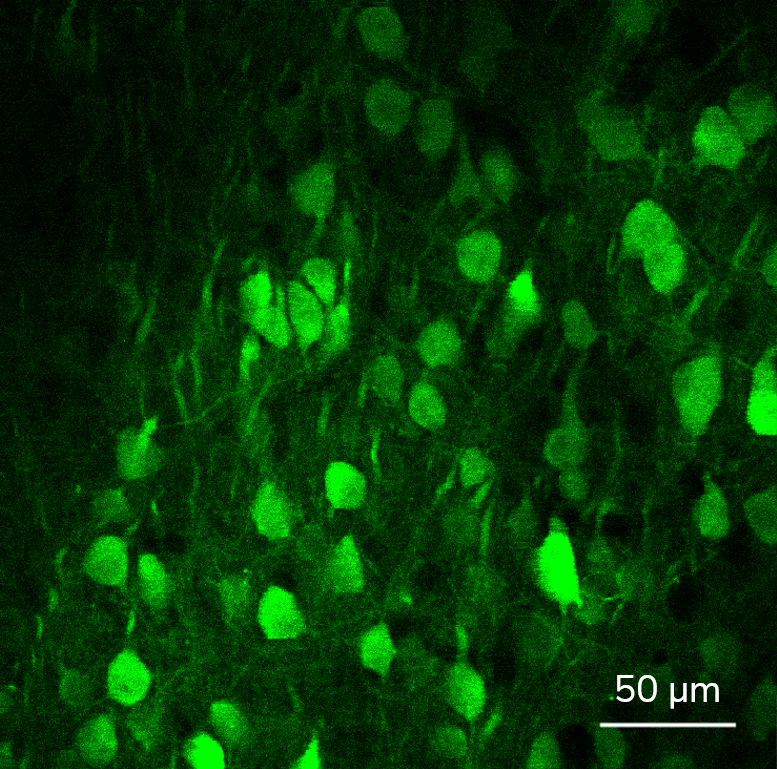
The figure shows spontaneous neuronal activity in the GCaMP6f-labeled visual cortex of a mouse captured using 3P microscopy: the cells were excited using a 1300 / 1400 nm laser wavelength for 3P excitation.
The 3P range microscopy kit contains:
- A pump laser and a Noncollinear Optical Parametric Amplifier (NOPA)
- High-quality optical elements optimized for transmission between 1,200-1,700 nm (with optional extension: get in contact with us for details)
- Full optical engineering
Features
- Excitation wavelength range: 1,200-1,700 nm
- XY resolution is diffraction-limited (less than 1 µm, wavelength-dependent), Z resolution ~ 5-10 µm (sample and wavelength-dependent)
-
Excites fluorophores with the 3P excitation phenomenon: 3P wavelength range (1,200-1,700 nm) corresponds to the regular (1P) excitation wavelength range of fluorophores ranging from blue to red
- Less tissue damage compared to 2P excitation
Benefits
- Functional and structural imaging through the whole cortical depth; provides an advantage in strongly scattering samples
- Third Harmonic Generation (THG) option is available
LED LIGHT SOURCE
for full-field photostimulation
/Compatible with FEMTO3D Atlas and FEMTOSmart/
Technology Overview
Full-field illumination using a selected LED source allows molecules and cells to be stimulated over the whole FOV homogeneously. Combine this module with gated detectors to achieve millisecond switching between stimulation and imaging.
See more at Optogenetics
Features
- Typical wavelengths: 430, 450, 480, 590 nm, others on special request
- Precisely timed and highly repeatable impulses
- ~200 µm penetration depth
- Software controlled operation
- Equipped on the objective arm
- PMT protection during the stimulation by optional gated detectors
Benefits
- Simultaneous stimulation over the entire FOV
- Optimized for optogenetics studies: precise Spatio-temporal activation of ChR2 and/or NpHR
- Millisecond switching between stimulation and detection
GREEN ILLUMINATION
for vessel pattern visualization
/Compatible with FEMTO3D Atlas and FEMTOSmart/
Technology Overview
Green illumination from an LED light source allows high-contrast visualization of blood vessels, taking advantage of that the highly concentrated heme content of the red blood cells is excitable in the spectral region of 300-650 nm. Using green illumination helps to navigate on the surface of any organs under in vivo conditions and position a patch pipette for bulk loading or patch clamping.
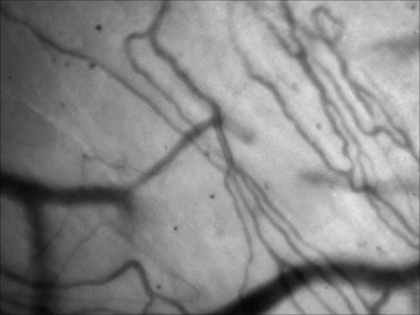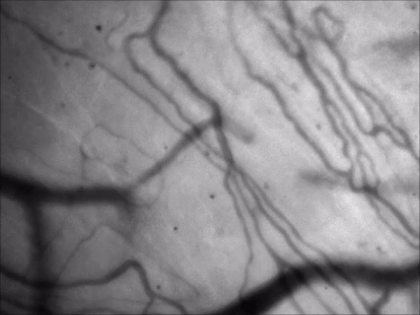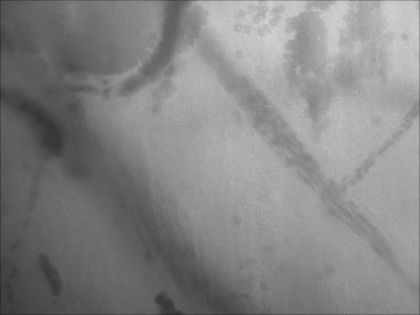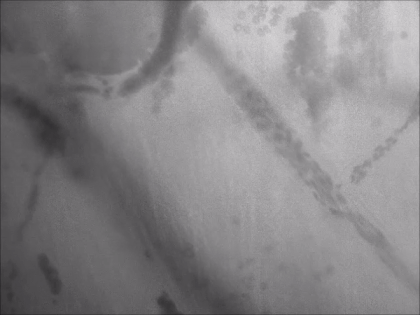
Features
- Excitation wavelength at 510-540 nm
- ~100 µm penetration depth
- 3 sec switching time between camera and two-photon modes
- Detection by CMOS camera
- Adjustable brightness, contrast and gamma parameters
- Software controlled operation
- Equipped on the objective arm
- Built-in PMT protection during operation
Benefits
- High-contrast visualization
- Precise overlap in XYZ between camera and two-photon recorded field of view
- Locate your chronic imaging spot within the craniotomy
4D Beam Conditioning Unit
for high-precision experiments
/Compatible with FEMTO3D Atlas/
Technology Overview
Beam stability is of the utmost importance, when it comes to accuracy in any imaging system. This is why we developed the 4DBCU (4D Beam Conditioning Unit), to efficiently deal with stabilization problems, and control the beam in an elegant and effective manner. This is a very efficient optional module for the FEMTO3D Atlas, one that will eliminate many optical artifacts, as well as prepare the beam for any specific application, such as deep functional imaging.
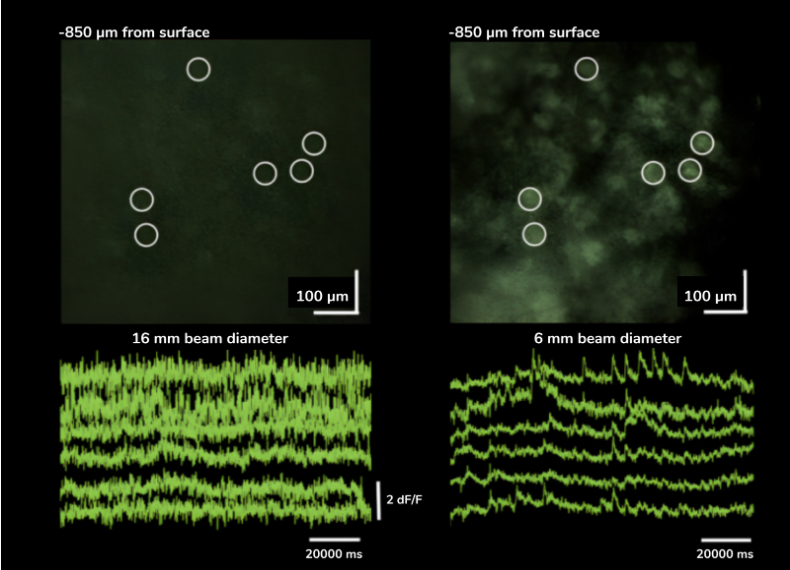
Features
- Compensates large temporal dispersion
- Automatic, motorized tuning on a broad wavelength and GDD range
- No alignment required after installation
- Compact design
- Includes collimator, beam stabilization unit, beam expander, which can be motorized to control the beam diameter at the back aperture of the objective precisely
- Precise control of effective excitation numerical aperture
Benefits
- Less Dispersion
- Controlled Diameter
- Smaller Divergence
- Negligible Displacement

FEMTO3D Atlas
The FEMTO3D Atlas software:
- our measurement control and analysis software suite powering the FEMTO3D Atlas,
- supports a multitude of imaging workflows from fast planar imaging to patterned 3D photostimulation and simultaneous multicolor activity recording.
Find 3D scanning modes and other features on the Atlas Software product page.

FEMTOSmart
The FEMTOSmart software:
- powers our conventional 2D two-photon microscopes,
- offers streamlined simplicity for efficient, standardized data collection,
- offers all the essential functions for conventional experimental scenarios, including:
- fast planar and Z-stack recordings,
- scanning along 2D trajectories,
- photostimulation,
- a compact set of tools for data visualization and analysis,
- hardware controls arranged in an ergonomically designed interface.

Analysis, programming tool, automation
- Our microscopes can be extended by optional modules implementing specific measurement or analysis functions.
- Extending or automating available acquisition control and analysis functions is possible by interfacing custom scripts to Atlas or FEMTOSmart microscopes.
HEAD HOLDERS
for rodents
Technology Overview
Head holder stands and head plates fix the rodent’s head in different positions, enabling precise measurements in the brain. Three types of head holders are available with different dimensions. For anesthetized rodents, we offer a heating pad coupled to a holder, or for behaving animals a stand that ensures access to jetballs, treadmills, or other devices. See more at Behavioral Studies.
 Flexible head holder
Flexible head holder
Offering maximum flexible adjustment for positioning the head, this holder fixes the scull from one side. The head plate can be moved in three directions of the space and also along the bridge. It is offered for difficult to access parts such as eyes or auditory cortex, and anesthetized rodents.
 Stabilized head holder
Stabilized head holder
Stabilized head holder is attached to the rodent’s head at two sides. It is ideal for craniotomy or other surgery where the head of the animal model has to be fixed. It can be coupled to the Gramophone.
Provided by
DNI-GLU-TFA
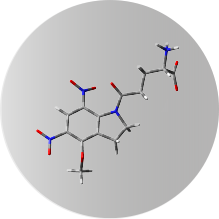 Technology Overview
Technology Overview
This dinitro-indoline-masked form of glutamate releases the bioactive glutamate more rapidly than any other commercially available compound. It was developed for high-quantum yield requiring less irradiation for release, so its effective concentration is lower than other caging scaffolds. The caged compound exists as trifluoroacetic acid salted form (DNI-Glu*TFA) ensuring good solubility, stability and low hygroscopicity. DNI-Glu is a compound developed in-house, only available from Femtonics.
See more at Uncaging.
Features
- Photostimulation with two-photon laser
- High quantum-yield
- Effective at low concentration
- Seven-fold higher quantum yield than any other caged form
- Stable and soluble
- Primarily used for in vitro experiments
Benefits
-
- High excitatory postsynaptic potential and high Ca2+ transient as a response to the photo release
- Less illumination is sufficient to elicit the same response as with alternative compounds
- Elicits large transients or regenerative activity
- Applicable for receptor mapping experiments
References
Endocannabinoid Signaling Mediates Local Dendritic Coordination between Excitatory and Inhibitory Synapses.
Hai Yin Hu, Dennis L.H. Kruijssen, Cátia P. Frias, Balázs Rózsa, Casper C. Hoogenraad, Cell Reports (2019)
A compact holographic projector module for high-resolution 3D multi-site two-photon photostimulation.
Mary Ann Go, Max Mueller, Michael Lawrence Castañares, Veronica Egger, Vincent R. Daria, PLOS One (2019)
Coincidence Detection within the Excitable Rat Olfactory Bulb Granule Cell Spines.
S. Sara Aghvami, Max Müller, Babak N. Araabi and Veronica Egger, J. Neurosci. (2019)
Voltage Gated Calcium Channel Activation by Backpropagating Action Potentials Downregulates NMDAR Function
Anne-Kathrin Theis, Balázs Rózsa, Gergely Katona, Dietmar Schmitz and Friedrich W. Johenning, Front. Cell. Neurosci. (2018)
High efficiency two-photon uncaging coupled by the correction of spontaneous hydrolysis.
Dénes Pálfi, Balázs Chiovini, Gergely Szalay, Attila Kaszás, Gergely F. Turi, Gergely Katona, Péter Ábrányi-Balogh, Milán Szőri, Attila Potor, Orsolya Frigyesi, Csilla Lukácsné Haveland, Zoltán Szadai, Miklós Madarász, Anikó Vasanits-Zsigrai, Ibolya Molnár-Perl, Béla Viskolcz, Imre G. Csizmadia, Zoltán Mucsi and Balázs Rózsa, Org. Biomol. Chem. (2018)
Super-Resolution Imaging of the Extracellular Space in Living Brain Tissue
Jan Tønnesen, V.V.G. Krishna Inavalli, U. Valentin Nägerl, Cell (2018)
Imaging membrane potential changes from dendritic spines using computer-generated holography.
Dimitrii Tanese, Ju-Yun Weng, Valeria Zampini, Vincent de-Sars, Marco Canepari, Balazs J. Rozsa, Valentina Emiliani, Dejan Zecevic, Neurophotonics (2017)
Cell-type–specific inhibition of the dendritic plateau potential in striatal spiny projection neurons.
Kai Du, Yu-Wei Wu, Robert Lindroos, Yu Liu, Balázs Rózsa, Gergely Katona, Jun B. Ding, and Jeanette Hellgren Kotaleski, PNAS (2017)
Electrical behaviour of dendritic spines as revealed by voltage imaging.
Marko A. Popovic, Nicholas Carnevale, Balazs Rozsa; Dejan Zecevic, Nature Communications (2015)
Local Postsynaptic Voltage-Gated Sodium Channel Activation in Dendritic Spines of Olfactory Bulb Granule Cells.
Wolfgang G. Bywalez, Dinu Patirniche, Vanessa Rupprecht, Martin Stemmler, Andreas;V.M. Herz, Denes Palfi, Balazs Rozsa, Veronica Egger, Neuron (2015)
Quantitation of various indolinyl caged glutamates as their o-phthalaldehyde derivatives by high performance liquid chromatography coupled with tandem spectroscopic detections: derivatization, stoichiometry and stability studies.
Vasanits-Zsigrai A, Majercsik O, Toth G, Csampai A, Haveland-Lukacs C, Palfi D, Szadai Z, Rozsa B, Molnar-Perl I, J Chromatogr A. (2015)
Dendritic spikes induce ripples in parvalbumin interneurons during hippocampal sharp waves.
B Chiovini, G F Turi, G Katona, A Kaszas, D Palfi, P Maak, G Szalay, M F Szabo, Z Szadai, Sz Kali and B Rozsa, Neuron (2014)
Provided by
DNI-Gly*TFA
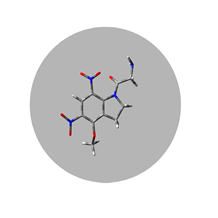 Technology Overview
Technology Overview
Femtonics Chemistry designs and develops new caged neurotransmitters for frontier neuroscience research. The two main products are a glutamate derivative and a GABA (gamma-amino-butyric acid) derivative. These dinitro-indoline-masked forms of glutamate and GABA release the bioactive forms of the two neurotransmitters more rapidly than other, commercially available versions of these compounds. They were developed to have high-quantum yield, requiring less irradiation for release, so their effective concentrations are lower than that of other caging scaffolds. DNI-Glu and iDMPO-DNI-GABA are compounds developed in-house, only available from Femtonics; in addition, iDMPO-DNI-GABA is the only commercially available caged GABA compound.
Features
- Name: 2-amino-1-(4-methoxy-5,7-dinitroindolin-1-yl)ethan-1-one trifluoroacetate
- Molecular formula: C13H13N4O8F3
- MW: 410.24 Da
- Standard packaging size: 12,5 mg (custom packaging available 6 mg -20 mg)
Benefits
-
- Rapidly and efficiently releases Gly (Glycine) neurotransmitter, by the effect of one (360 nm) or two photon (720 nm) irradiation
- Glycine is an inhibitory neurotransmitter on GlyR in the CNS, especially in the spinal cord, brainstem, and retina, via ionotropic receptors, causing an Inhibitory postsynaptic potential (IPSP)
- Exists as trifluoroacetic acid salted form, ensuring good solubility, stability and low hygroscopicity of the product
- Highly resistant to hydrolysis at neutral pH
- High quantum yield
References
Molecular structure and function of the glycine receptor chloride channel.
Lynch, J. W., Physiological Reviews (2004)
Provided by
DNI-NMDA*TFA
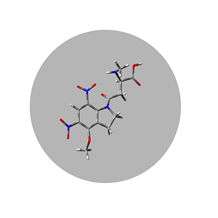 Technology Overview
Technology Overview
Femtonics Chemistry designs and develops new caged neurotransmitters for frontier neuroscience research. The two main products are a glutamate derivative and a GABA (gamma-amino-butyric acid) derivative. These dinitro-indoline-masked forms of glutamate and GABA release the bioactive forms of the two neurotransmitters more rapidly than other, commercially available versions of these compounds. They were developed to have high-quantum yield, requiring less irradiation for release, so their effective concentrations are lower than that of other caging scaffolds. DNI-Glu and iDMPO-DNI-GABA are compounds developed in-house, only available from Femtonics; in addition, iDMPO-DNI-GABA is the only commercially available caged GABA compound.
Features
- Name: 4-methoxy-5,7-dinitroindolinyl-N-methyl-D-aspartate trifluoroacetate
- Molecular formula: C16H17N4O10F3
- MW: 482.32 Da
- Standard packaging size: 6 mg (custom packaging available 14 mg or 20 mg )
Benefits
-
- Rapidly and efficiently releases NMDA (N-methyl-D-Asp) neurotransmitter (selective NMDAR agonist), by the effect of one (360 nm) or two photon (720 nm) irradiation
- Exists as trifluoroacetic acid salted form, ensuring good solubility, stability and low hygroscopicity of the product
- Highly resistant to hydrolysis at neutral pH
- Higher quantum yield (ca. 7 times, than MNI-NMDA)
- Releases NMDA neurotransmitter more rapidly by the effect of two photon irradiation (720 nm) than MNI-NMDA
References
Stereotyped initiation of retinal waves by bipolar cells via presynaptic NMDA autoreceptors.
Zhang, R. W. et al. Nat. Commun. (2016)
New caged neurotransmitter analogs selective for glutamate receptor sub-types based on methoxynitroindoline and nitrophenylethoxycarbonyl caging groups.
Palma-Cerda, F. et al. Neuropharmacology (2012)
Provided by
DNI-D-Asp*TFA
 Technology Overview
Technology Overview
Femtonics Chemistry designs and develops new caged neurotransmitters for frontier neuroscience research. The two main products are a glutamate derivative and a GABA (gamma-amino-butyric acid) derivative. These dinitro-indoline-masked forms of glutamate and GABA release the bioactive forms of the two neurotransmitters more rapidly than other, commercially available versions of these compounds. They were developed to have high-quantum yield, requiring less irradiation for release, so their effective concentrations are lower than that of other caging scaffolds. DNI-Glu and iDMPO-DNI-GABA are compounds developed in-house, only available from Femtonics; in addition, iDMPO-DNI-GABA is the only commercially available caged GABA compound.
Features
- Name: 4-methoxy-5,7-dinitroindolinyl-D-aspartate trifluoroacetate
- Molecular formula: C15H15N4O10F3
- MW: 468.30 Da
- Standard packaging size: 6 mg (custom packaging available 14 mg or 20 mg)
Benefits
-
- Rapidly and efficiently releases D-Asp neurotransmitter, by the effect of one (360 nm) or two photon (720 nm) irradiation
- Agonist at NMDA receptors and EAAT substrate
- Exists as trifluoroacetic acid salted form, ensuring good solubility, stability and low hygroscopicity of the product
- Highly resistant to hydrolysis at neutral pH
- Higher quantum yield (ca. 7 times, than MNI-D-Asp)
- Releases D-Asp neurotransmitter more rapidly by the effect of two photon irradiation (720 nm) than MNI-D-Asp
References
Synthesis and characterization of 4-methoxy-7-nitroindolinyl-D-aspartate, a caged compound for selective activation of glutamate transporters and N-MthD.-aspartate receptors in brain tissue.
Huang, Y. H. et al. Biochemistry (2005)
Provided by
iDMPO-DNI-GABA*TFA
Technology Overview
Femtonics Chemistry designs and develops new caged neurotransmitters for frontier neuroscience research. The two main products are a glutamate derivative and a GABA (gamma-amino-butyric acid) derivative. These dinitro-indoline-masked forms of glutamate and GABA release the bioactive forms of the two neurotransmitters more rapidly than other, commercially available versions of these compounds. They were developed to have high-quantum yield, requiring less irradiation for release, so their effective concentrations are lower than that of other caging scaffolds. DNI-Glu and iDMPO-DNI-GABA are compounds developed in-house, only available from Femtonics; in addition, iDMPO-DNI-GABA is the only commercially available caged GABA compound.
Features
- Name: 4-aminoalkyl-5,7-dinitroindolinyl-GABA trifluoroacetate
- Molecular formula: C21H27N5O10F6
- MW: 623.50 Da
- Standard packaging size: 19 mg (custom packaging available 6 mg -20 mg)
Benefits
-
- Rapidly and efficiently releases GABA (γ-aminobutyric acid) neurotransmitter, by the effect of one (360 nm) or two photon (720 nm) irradiation
- GABA is the chief inhibitory neurotransmitter in the mammalian central nervous system. Its principal role is reducing neuronal excitability throughout the nervous system
- Exists as trifluoroacetic acid salted form, ensuring good solubility, stability and low hygroscopicity of the product
- Highly resistant to hydrolysis at neutral pH
- High quantum yield
References
Theoretical Design, Synthesis, and In Vitro Neurobiological Applications of a Highly Efficient Two-Photon Caged GABA Validated on an Epileptic Case.
Chiovini, B. et al. ACS Omega (2021)
Disruption of centrifugal inhibition to olfactory bulb granule cells impairs olfactory discrimination.
Nunez-Parra, A. et al. PLoS One (2013)
Presynaptic miniature GABAergic currents in developing interneurons.
Trigo, F. F. et al. Neuron (2010)
Laser photolysis of DPNI-GABA, a tool for investigating the properties and distribution of GABA receptors and for silencing neurons in situ.
Trigo, F. F. et al. J.Neurosci.Meths. (2009)
GABA and GABA receptors in the central nervous system and other organs.
Watanabe, M. et al. Int. Rev. Cytol. (2002)
Provided by
GFZnP-ED
 Technology Overview
Technology Overview
As the second most common transition metal in the body, the detection of Zn2+ is a necessity in many fields of applications. It has been related to neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, or to important viral strains, such as the SARS-Cov-2. Therefore, we have developed three brand new fluorescent Zn2+ sensors, which have the capacity for spatial and temporal monitoring, which has not been the case with previous methods. These are sensors for one and two-photon microscopy, which are the two most available, sensitive and inexpensive methods for Zn2+ monitoring, with the aid of the high solubility, high fluorescens and optimal selectivity.
Features
- Name: (Z)-5-((8-((2-(dimethylamino)ethyl)(methyl)amino)quinolin-2-yl)methylene)-2-(phenylamino)-3,5-dihydro-4H-imidazol-4-one
- Molecular formula: C24H26N6O
- MW: 414.50 Da
- Standard packaging size: 250 µL, 5 mM
Benefits
-
- Highest dynamic range (ΔF/F0 = 77) of our Zn2+ probes in one-photon setups
- The excitation wavelength is 450 nm, while the emission is 509 nm.
- The 2P-excitation wavelength is 900 nm
References
Two-photon fluorescent chemosensors based on the GFP-chromophore for the detection of Zn2+ in biological samples – From design to application
A. Csomos, E. Kovács, M. Madarász, F. Zs. Fedor, A. Fülöp, G. Katona, B. Rózsa, Z. Mucsi, Sensors and Actuators B: Chemical (2023)
Provided by
GFZnP-Pic
 Technology Overview
Technology Overview
As the second most common transition metal in the body, the detection of Zn2+ is a necessity in many fields of applications. It has been related to neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, or to important viral strains, such as the SARS-Cov-2. Therefore, we have developed three brand new fluorescent Zn2+ sensors, which have the capacity for spatial and temporal monitoring, which has not been the case with previous methods. These are sensors for one and two-photon microscopy, which are the two most available, sensitive and inexpensive methods for Zn2+ monitoring, with the aid of the high solubility, high fluorescens and optimal selectivity.
Features
- Name: (Z)-5-((8-(methyl(pyridin-2-ylmethyl)amino)quinolin-2- yl)methylene)-2-(phenylamino)-3,5- dihydro-4H-imidazol-4-one
- Molecular formula: C26H22N6O
- MW: 434.50 Da
- Standard packaging size: 250 µL, 5 mM
Benefits
-
- Brightest sensor for 2P experiments
- The best dynamic range
- The excitation wavelength is 444 nm, while the emission is 503 nm
- The 2P-excitation wavelength is 900 nm
References
Two-photon fluorescent chemosensors based on the GFP-chromophore for the detection of Zn2+ in biological samples – From design to application
A. Csomos, E. Kovács, M. Madarász, F. Zs. Fedor, A. Fülöp, G. Katona, B. Rózsa, Z. Mucsi, Sensors and Actuators B: Chemical (2023)
Provided by
GFZnP-diPic
 Technology Overview
Technology Overview
As the second most common transition metal in the body, the detection of Zn2+ is a necessity in many fields of applications. It has been related to neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, or to important viral strains, such as the SARS-Cov-2. Therefore, we have developed three brand new fluorescent Zn2+ sensors, which have the capacity for spatial and temporal monitoring, which has not been the case with previous methods. These are sensors for one and two-photon microscopy, which are the two most available, sensitive and inexpensive methods for Zn2+ monitoring, with the aid of the high solubility, high fluorescens and optimal selectivity.
Features
- Name: (Z)-5-((8-(bis(pyridin-2- ylmethyl)amino)quinolin-2- yl)methylene)-2-(phenylamino)-3,5- dihydro-4H-imidazol-4-one
- Molecular formula: C31H25N7O
- MW: 511.59 Da
- Standard packaging size: 250 µL, 5 mM
Benefits
-
- our most sensitive sensor for Zn2+
- With an exceptional affinity coefficient of K’d = 0.15 nM
- The excitation wavelength is 441 nm, while the emission is 503 nm
- The 2P-excitation wavelength is 900 nm
References
Two-photon fluorescent chemosensors based on the GFP-chromophore for the detection of Zn2+ in biological samples – From design to application
A. Csomos, E. Kovács, M. Madarász, F. Zs. Fedor, A. Fülöp, G. Katona, B. Rózsa, Z. Mucsi, Sensors and Actuators B: Chemical (2023)
Technology Overview
This dinitro-indoline-masked form of glutamate releases the bioactive glutamate more rapidly than any other commercially available compound. It was developed for high-quantum yield requiring less irradiation for release, so its effective concentration is lower than other caging scaffolds. The caged compound exists as trifluoroacetic acid salted form (DNI-Glu*TFA) ensuring good solubility, stability and low hygroscopicity. DNI-Glu is a compound developed in-house, only available from Femtonics.
Features
- Photo stimulation with two-photon laser
- High quantum-yield
- Effective at low concentration
- Seven-fold higher quantum yield than any other caged form
- Stable and soluble
- Primarily used for in vitro experiments
Benefits
- High excitatory postsynaptic potential and high Ca2+ transient as a response to the photo release
- Less illumination is sufficient to elicit the same response as with alternative compounds
- Elicits large transients or regenerative activity
- Applicable for receptor mapping experiments
Introduction
Lorem ipsum dolor sit amet consectetur, adipisicing elit. Tenetur deleniti architecto, optio quibusdam cum, rerum, beatae molestiae eos saepe perspiciatis quos voluptates ad. Fugiat ducimus dignissimos non, illo eligendi esse?

Solution
Lorem ipsum dolor sit amet consectetur, adipisicing elit. Tenetur deleniti architecto, optio quibusdam cum, rerum, beatae molestiae eos saepe perspiciatis quos voluptates ad. Fugiat ducimus dignissimos non, illo eligendi esse?
Lorem ipsum dolor sit amet consectetur adipisicing elit. Laborum, magnam nesciunt, sapiente quas molestiae fugiat placeat similique quisquam cum omnis culpa ullam reiciendis ab cupiditate eveniet laudantium animi maiores impedit!
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Maxime similique, amet aperiam dolores sit nisi unde debitis, adipisci eos illum suscipit, nemo eligendi magni laudantium quos labore modi esse delectus.

EXTREMELY LARG SPACE UNDER THE OBJECTIVE FEMTO SMART MICROSCOPES
Lorem ipsum dolor sit amet consectetur, adipisicing elit. Tenetur deleniti architecto, optio quibusdam cum, rerum, beatae molestiae eos saepe perspiciatis quos voluptates ad. Fugiat ducimus dignissimos non, illo eligendi esse?

FEMTONICS GRAMOPHONE
Lorem ipsum dolor sit amet consectetur, adipisicing elit. Tenetur deleniti architecto, optio quibusdam cum, rerum, beatae molestiae eos saepe perspiciatis quos voluptates ad. Fugiat ducimus dignissimos non, illo eligendi esse?
Lorem ipsum dolor sit amet consectetur adipisicing elit. Laborum, magnam nesciunt, sapiente quas molestiae fugiat placeat similique quisquam cum omnis culpa ullam reiciendis ab cupiditate eveniet laudantium animi maiores impedit!
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Maxime similique, amet aperiam dolores sit nisi unde debitis, adipisci eos illum suscipit, nemo eligendi magni laudantium quos labore modi esse delectus.


Femto3d
Atlas
All of our knowledge and results of acousto-optic technology have been poured into our new product, the Femto3D Atlas which has become the peak of the evolution of Femtonics acousto-optic microscopes. Atlas combines an intoxicating blend of high-tech science, engineering, refinement in 3D measurements. Using Atlas, researchers are capable of scanning neuronal, dendritic, and other neuropil activities in 3D about one million times faster compared to classical scanning methods with preserved two-photon resolution thanks to a unique acousto-optical scanner and the superior optical design. Combining its unique fast 3D imaging feature with the property that it implements and goes beyond the traditional galvo and resonant scanner-based microscope imaging functions, Atlas provides an all-in-one solution into the scientists’ hand.
FEATURES AND BENEFITS
- 3D imaging extension for any existing microscopes and individual system
- Simultaneous read out of neural activity on both the somatic and dendritic scales
- Increased measurement speed and signal-to-noise ratio by several orders of magnitude
- 3D Network imaging of over 2000 soma distributed in 3D
- Imaging of dendritic branches through layers
- Simultaneous imaging of over thousands of spines
- Unique, flexible scanning methods supporting in vivo and in vitro measurements
- In vivo 3D motion correction by specialized scanning patterns
- High-speed 3D random-access ROI scanning: scanning speed up to 30 kHz to any points in 3D
- High-speed raster scanning: 40 fps at 510x510, and even 3000 fps at reduced FOV
- Improved excitation efficiency for large 3D scanning volume with GECIs
- Automatic wavelength tuning between 750 - 1050 nm
- Functional imaging correlated with electrophysiological data
- Calcium imaging during behavior
- 3D random-access photo stimulation for optogenetics

Femto
Smart
The FemtoSmart series is the next step in classical two-photon microscopy at Femtonics, fully customizable two-photon microscopes. Their special feature is the elevated body which can move in X, Y, and Z directions, providing ample room under the objective for optimal positioning of your sample. This feature makes them suitable for model organisms ranging from zebra fish larvae, through mice navigating in virtual reality to even non-human primates. The microscope’s modular nature allows us to assemble the components, and recombine and upgrade the system to perfectly fit the customer’s needs. FemtoSmart product line contains galvo and/or resonant scanner-based systems which can be equipped with a lot of optional modules enabling it to be adapted to a wide range of biological applications, such as optogenetics, uncaging and dendritic imaging.
FEATURES AND BENEFITS
- Elevated, column-based body
- XYZ positioning
- Tilting objective
- High level of modularity
- Galvanometric, resonant, or both scanning possibilities
- Galvo Scanner, focusing on regions-of-interest
- Resonant Scanner, fast frame and volume scanning
- Dual Scanner, all advantages of Galvo and Resonant microscopes
- Travelling detector system
- For in vivo studies
- Field upgradability
- Patented imaging technologies
- Flexible scanning methods
- Maximal photon collection

 Flexible head holder
Flexible head holder Stabilized head holder
Stabilized head holder