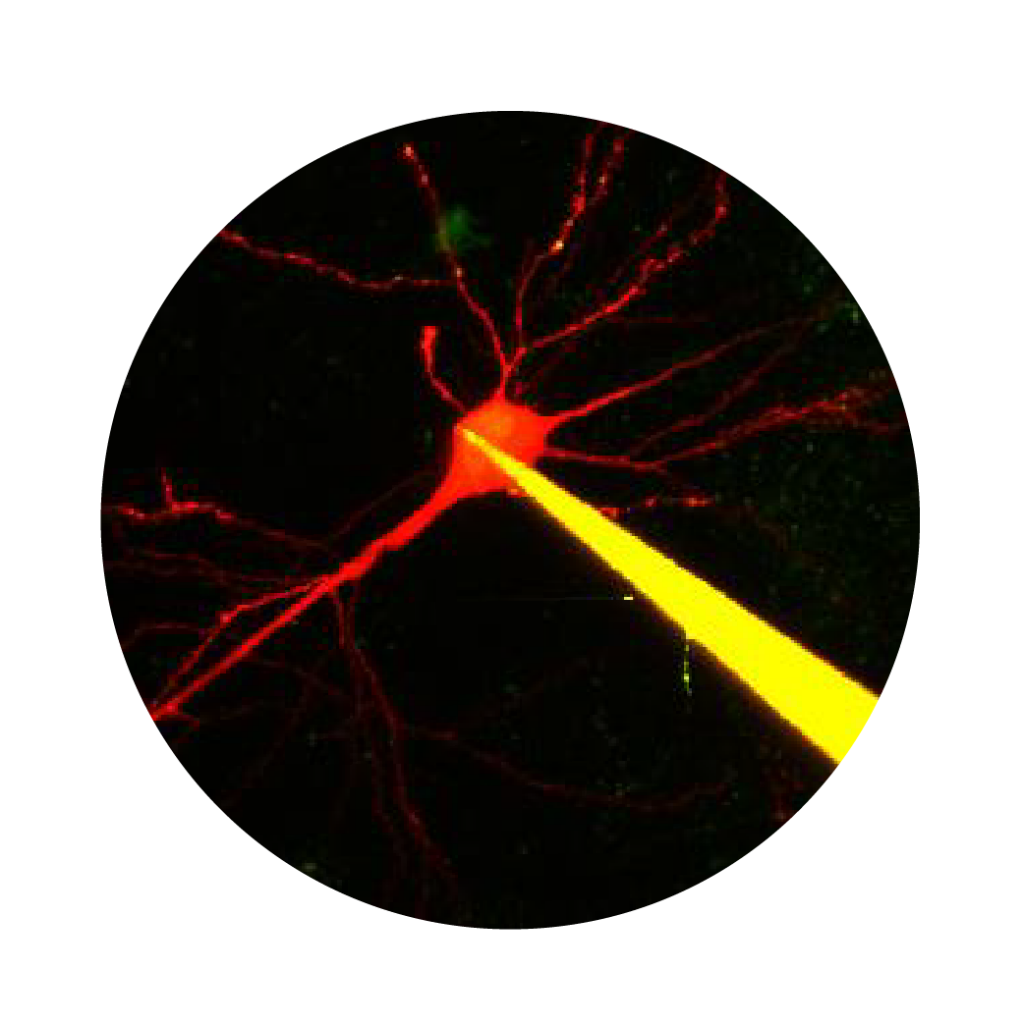
Electrophysiology
Two-photon Ca2+ imaging and electrophysiological data acquisition provide excellent tools for the study of neural cell and network activity. Using the two methods simultaneously, it is possible to follow potential fluctuations and relate them to changes in their Ca2+ levels. While the two-photon Ca2+ imaging technique shows events in multiple small areas (distinguishable parts of dendrites and spines) at high spatial resolution (less than 1 µm) but low temporal resolution (>100 ms), electrophysiology can cover changes over larger areas (cells or extensive areas) at low spatial resolution (~150 µm), and the changes can be followed considerably faster (<1 ms).
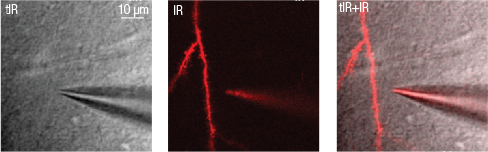
Dendritic patch-clamp
With intracellular patch-clamp recording it is possible to observe and manipulate membrane potential fluctuations in neurons. All Femtonics microscopes can be equipped with electrophysiological devices, and the following settings facilitate the experiments:
- automatic software module moves the tip of the pipette to the selected dendritic location,
- pipette movement and distance to target location can be monitored in real-time,
- guide points can be positioned by the user according to the scanned transmitted or two-photon images,
- real-time transmitted infrared and two photon images can be shown separately or in overlay,
- only one simple calibration step is required before using a new recording pipette.
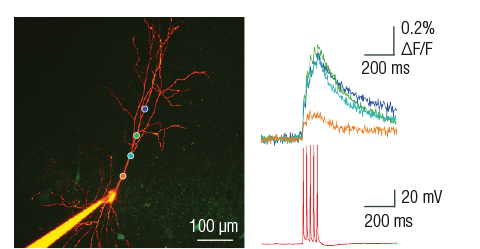
Processing of electrophysiological data
The electrophysiology software module acquires electrophysiology signals using the A/D converters of the microscope. The telegraphing function communicates the conversion parameters from the amplifier to the A/D converters managed by the control software. Electrophysiology signal acquisition time is aligned with the imaging data. The electrophysiology GUI also includes a seal test window.
The microscope is fully equipped with high-bandwith two-way communication ports realizing input and output options through BNC connected cables, providing both digital and analogue in- and outputs configured according to the needs of the future user constrained only by the total bandwith of the electronic communication and driver cards. Communication is fully mediated by the software through different modules starting from the protocol editor through modules allowing scripting.
References
Multiple Two-Photon Targeted Whole-Cell Patch-Clamp Recordings From Monosynaptically Connected Neurons in vivo. Jean-Sébastien Jouhanneau and James F. A. Poulet, Front. Synaptic Neurosci. (2019)
Voltage Gated Calcium Channel Activation by Backpropagating Action Potentials Downregulates NMDAR Function. Anne-Kathrin Theis, Balázs Rózsa, Gergely Katona, Dietmar Schmitz and Friedrich W. Johenning, Front. Cell. Neurosci. (2018)
Electrical behaviour of dendritic spines as revealed by voltage imaging. Marko A. Popovic, Nicholas Carnevale, Balazs Rozsa; Dejan Zecevic, Nature Communications (2015)
Dendritic spikes induce ripples in parvalbumin interneurons during hippocampal sharp waves. B Chiovini, G F Turi, G Katona, A Kaszas, D Palfi, P Maak, G Szalay, M F Szabo, Z Szadai, Sz Kali and B Rozsa, Neuron (2014)
Combined two-photon imaging, electrophysiological, and anatomical investigation of the human neocortex in vitro. Balint Peter Kerekes, Kinga Toth, Attila Kaszas, Balazs Chiovini, Zoltan Szadai, Gergely Szalay, Denes Palfi, Attila Bago, Klaudia Spitzer, Balazs Rozsa, Istvan Ulbert, Lucia Wittner, Neurophotonics (2014)
Dendritic spikes induce ripples in parvalbumin interneurons during hippocampal sharp waves. B Chiovini, G F Turi, G Katona, A Kaszas, D Palfi, P Maak, G Szalay, M F Szabo, Z Szadai, Sz Kali and B Rozsa, Neuron (2014)
Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Gergely Katona, Gergely Szalay, Pál Maak, Attila Kaszas, Mate Veress, Daniel Hillier, Balazs Chiovini, E Sylvester Vizi, Botond Roska & Balazs Rozsa, Nature Methods (2012)
Enhanced Dendritic Action Potential Backpropagation in Parvalbumin-positive Basket Cells During Sharp Wave Activity. B. Chiovini, GF. Turi, G. Katona, A. Kaszas, F. Erdelyi, G. Szabo, H. Monyer, A. Csakanyi, ES. Vizi, B. Rozsa, Neurochemical Research (2010)
