Behavioral studies
The Central Nervous System organizes behavior, therefore to study CNS functions works best in a behavioral context. In this context it is important to manipulate experimental variables in a precisely controlled way. Those variables may be stimulus information, task instructions, reward or aversive outcome, all affecting the related pattern of brain activation. Monitoring and manipulating behavior together with imaging neural network activity in the awake behaving animal is essential to establish the relation between the cellular/network and behavioral level.
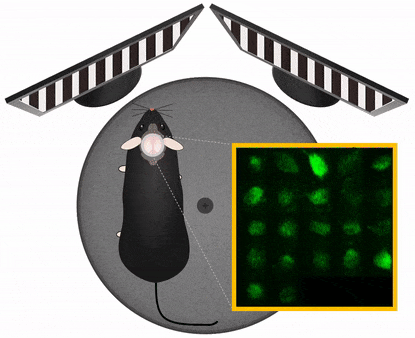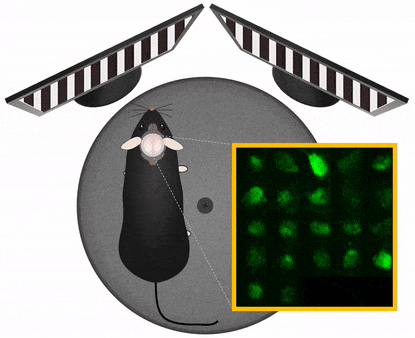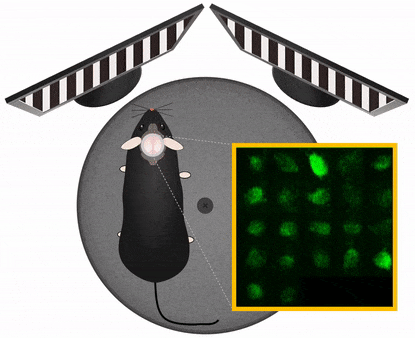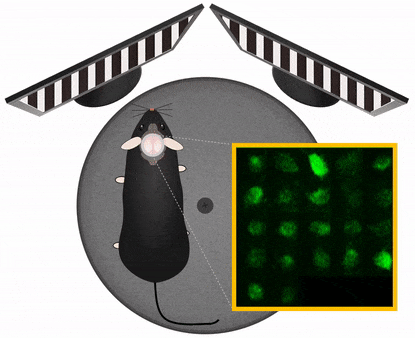


Movement artefact correction
Imaging cellular activity in behaving animals is challenging, since all kinds of movement may generate imaging artifacts additional to that caused by physiological processes like heartbeat and breathing. The presence of physical activity can particularly complicate data collection within the specific timeframe of interest; As the activity of interest is closely related to the movement as behavioral marker, it is very important to be able to keep the recording clean of movement artifacts in a behavioral experiment.
Femtonics FocusPinner: 3D Real-Time Motion Correction solution
With our truly 3D, blazing-fast motion correction solution, Femtonics 3D Real-Time Motion Correction, FEMTO3D Atlas imaging platform can follow the displacement of the ROIs during the experiments eliminating artifacts caused by heartbeat, breathing or physical activity allowing calcium and voltage imaging from dendrites and somata with high resolution.
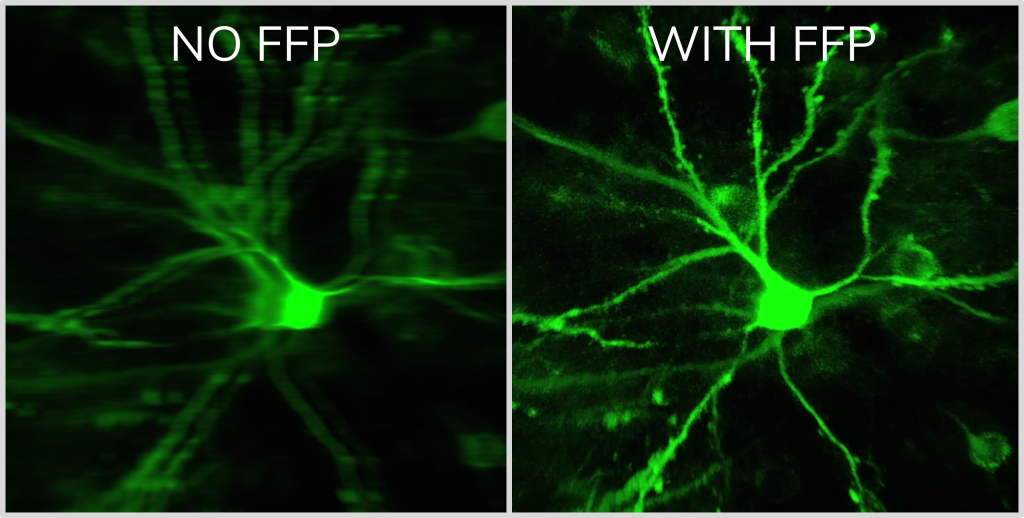
Surface and volume scanning possibilities supporting motion correction
Adjacent drifts may cover surface-like recording regions, surfaces can be extended to form volumes. This way areas or volumes around the objects of interest can be recorded thereby providing basis for a post-recording motion correction allowing to preserve physiological data despite tissue movements. (Szalay et. al, Neuron (2016). Post-hoc movement correction algorithms built in the software ensure subresolution alignment of ROIs even at large artifacts in 3D.
The surface scanning methods are optimized for speed, while the methods based on volume imaging are optimized for large amplitude movements. Each scanning mode is useful for different neurobiological aims: ribbon scanning, snake scanning, 3D multiple line scanning areis optimal for various dendritic measurements, while chessboard scanning and multi-cube scanning are best used for somatic recordings.

FemtoSmart offers numerous imaging opportunities and thanks to its modularity researchers can study dendrites with spines from wide variations of perspectives.
The fastest in 2D
2D Multiple-Line Scanning by FEMTOSmart GALVO
The adaptable X and Y mirrors of the galvanometric scanner, coupled with the special electronic boards from Femtonics, can follow the tortuous protrusions of the dendritic arbor precisely using the 2D random-access point, multiple-line and folded frame scanning methods. By limiting the scanning to the interesting dendritic segments carrying spines and omitting the space between them, both the scanning speed (up to 2 kHz) and the SNR can be increased using 2D random-access point and multiple-line scanning modes. The advantage is multiple-line scanning is the imaging without interruption at multiple dendritic branches, it is the cost-effective, alternative solution compared to the acousto-optic scanner-based line scanning methods.
Imaging dendrites and spines with motion correction
Folded frame scanning by FEMTOSmart GALVO
Using folded frame scanning, an area along a pre-selected line can be imaged. The selected regions can take many shapes, from areas around straight lines to complex bent curves. This advanced scanning method is useful for following events along curved dendrites with spines, and can also be advantageous for dendritic measurements in behaving animal models where motion artefacts are a common problem. The images are corrected for motion offline by the control software, as long as the dendrite remains in the scanned area.
Coming Soon: Automated dendrite selection tool
As dendritic segments are abundant, complex, 3 dimensional features of the brain, selecting ROIs around them is a maticulous job, reliant on human perception. With the help of this optional module, you will be able select all dendritic segments in your field of view, just with some push of buttons. This will save you time and energy, and the end result will be more precisely aligned, down to the pixel level!
References
Pyramidal neurons form active, transient, multilayered circuits perturbed by autism-associated mutations at the inception of neocortex Munz M, Bharioke A, Kosche G, Moreno-Juan V, Brignall A, Rodrigues TM, Graff-Meyer A, Ulmer T, Haeuselmann S, Pavlinic D, Ledergerber N, Gross-Scherf B, Rózsa B, Krol J, Picelli S, Cowan CS, Roska B
Fast 3D Imaging of Spine, Dendritic, and Neuronal Assemblies in Behaving Animals. Gergely Szalay, Linda Judak, Gergely Katona, Katalin Ocsai, Gabor Juhasz, Mate Veress, Zoltan Szadai, Andras Feher, Tamas Tompa, Balazs Chiovini, Pal Maak, Balazs Rozsa, Neuron (2016)
Dendritic spikes induce ripples in parvalbumin interneurons during hippocampal sharp waves. B Chiovini, G F Turi, G Katona, A Kaszas, D Palfi, P Maak, G Szalay, M F Szabo, Z Szadai, Sz Kali and B Rozsa, Neuron (2014)
Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Gergely Katona, Gergely Szalay, Pál Maak, Attila Kaszas, Mate Veress, Daniel Hillier, Balazs Chiovini, E Sylvester Vizi, Botond Roska & Balazs Rozsa, Nature Methods (2012)
Matching Cell Type to Function in Cortical Circuits. Luc Estebanez, Jens Kremkow, James F.A. Poulet, Neuron (2015)
In Vivo Monosynaptic Excitatory Transmission between Layer 2 Cortical Pyramidal Neurons. Jean-Sebastien Jouhanneau, Jens Kremkow, Anja L. Dorrn, James F.A. Poulet, Cell Reports (2015)
Roller Coaster Scanning reveals spontaneous triggering of dendritic spikes in CA1 interneurons. G. Katona, A. Kaszas, G. F. Turi, N. Hajos, G. Tamas, E.S. Vizi, B. Rozsa, PNAS (2011)
