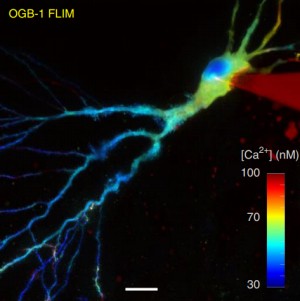
Fluorescence-lifetime imaging microscopy – FLIM
Fluorescence-lifetime imaging microscopy (FLIM) delivers both structural (intensity image) and functional (lifetime image) information and minimizes the imaging effect of photon scattering in thick layers of sample. FLIM provides an accurately quantitative assessment of changes of many biophysical parameters in a target molecular microenvironment, without being affected by the intensity of the emission, quenching or uneven fluorophore distribution. The most frequent FLIM applications are the measurement of molecular environment parameters, molecular interaction and distance measurements by Förster resonance energy transfer (FRET), besides studying the metabolic state of cells and tissues via their autofluorescence. Two-photon fluorescence-lifetime imaging microscopy (2P-FLIM) meets the needs of neuroscientific research because of the combined advantages of two-photon excitation and fluorescence-lifetime imaging: superior depth penetration and decreased photo-bleaching, owing to long excitation wavelengths, and inherent optical sectioning.
The FEMTOSmart for FLIM
The FEMTOSmart Galvo upgraded with the FLIM extension is suitable for performing in vivo and in vitro 2P-FLIM studies in a wide range of model organisms. In this complex system, up to two hybrid detectors (combination of GaAsP cathode and an avalanche detector) with two photon counting channels provide a high detection efficiency and a fast timing response, with less noise. As afterpulsing is essentially absent in these detectors, the dynamic range of fluorescence decay recordings is considerably increased. The special scanning modes and patterns of the FEMTOSmart Galvo provide high spatiotemporal resolution during measurements. The setup is perfectly suitable to monitor glutamate release and intracellular calcium concentration, to perform FRET measurements, or to quantify the ratio between reduced and oxidized NADH and FAD molecules, in the research of neurodegenerative diseases or to reveal metabolic changes during tumorigenesis in cancer research.
Tornado imaging for the direct monitoring of single-quantum, single-synapse glutamate release in situ
A FEMTOSmart Galvo with a FLIM extension is suitable to follow single action potential-evoked presynaptic Ca2+ dynamics, with nanomolar sensitivity, in individual small, presynaptic boutons in acute brain slices. Jensen et al., from the laboratory of Prof. Dmitri Rusakov, have advanced a two-photon excitation FLIM technique. They used the flexible Tornado scanning mode of our galvanometric scanner-based microscope, which provides pixel sampling in a spiral, recording the signal’s spatiotemporal dynamics more favourably, and improves the signal-to-noise ratio. With this method, they performed time-resolved imaging of Ca2+ sensitive fluorescence lifetime of Oregon Green BAPTA-1. In parallel, intensity Tornado imaging made it possible to monitor single-quantum, single-synapse glutamate release in situ directly, using the locally expressed extracellular optical glutamate sensor iGluSnFr. This study paves the way for simultaneous registration of presynaptic Ca2+ dynamics and transmitter release in an intact brain at the level of individual synapses.
(Related article: Monitoring single-synapse glutamate release and presynaptic calcium concentration in organised brain tissue, Thomas P. Jensen, Kaiyu Zheng, Olga Tyurikova, James P. Reynolds, Dmitri A. Rusakov, Cell Calcium, 2017)
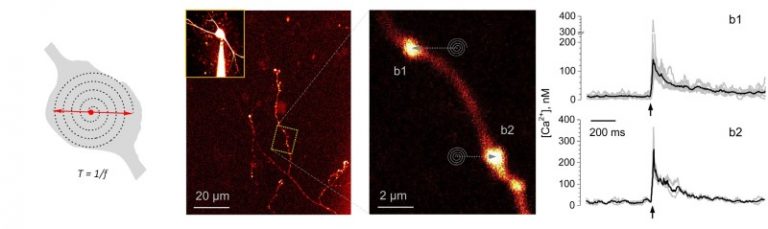
FLIM imaging reveals that distinct sites in individual boutons release glutamate in a probabilistic fashion
In a subsequent publication from the same laboratory, Rama et al. describe how they used the FEMTOSmart Galvo with the FLIM extension to monitor spike-evoked glutamate release and presynaptic calcium entry in the mossy fiber boutons of granule cells, with the use of the optical glutamate sensor SF-iGluSnFR and the intracellular Ca2+ indicator Cal-590, and the above mentioned Tornado scanning mode. These presynaptic boutons feature multiple release sites and are densely packed with synaptic vesicles to sustain a highly facilitating “detonator” transmission. In this study, multiplexed imaging showed that distinct sites in individual boutons release glutamate in a probabilistic fashion, while also showing use-dependent short-term facilitation. The results help to reveal whether glutamate release sites act independently or rather synchronously, and to understand the fundamentals of synaptic transmission and hippocampal network function in a wider context.
(Related article: Glutamate Imaging Reveals Multiple Sites of Stochastic Release in the CA3 Giant Mossy Fiber Boutons, Sylvain Rama, Thomas P. Jensen, Dmitri A. Rusakov, Frontiers in Cellular Neuroscience, 2019)

References
Fluorescence lifetime imaging reveals regulation of presynaptic Ca2+ by glutamate uptake and mGluRs, but not somatic voltage in cortical neurons. Olga Tyurikova, Kaiyu Zheng, Elizabeth Nicholson, Yulia Timofeeva, Alexey Semyanov, Kirill Volynski, Dmitri A. Rusakov, Journal of Neurochemistry (2020)
Local Resting Ca2+ Controls the Scale of Astroglial Ca2+ Signals. Claire M. King, Kirsten Bohmbach, Daniel Minge, Andrea Delekate, Kaiyu Zheng, James Reynolds, Cordula Rakers, Andre Zeug, Gabor C. Petzold, Dmitri A. Rusakov, Christian Henneberger, Cell Reports (2020)
Multiplex imaging relates quantal glutamate release to presynaptic Ca2+ homeostasis at multiple synapses in situ. Thomas P. Jensen, Kaiyu Zheng, Nicholas Cole, Jonathan S. Marvin, Loren L. Looger, Dmitri A. Rusakov, Nature Communications (2019)
Polymer microchamber arrays for geometry-controlled drug release: a functional study in human cells of neuronal phenotype. Olga Kopach, Kayiu Zheng, Olga A. Sindeeva, Meiyu Gai, Gleb B. Sukhorukov, Dmitri A. Rusakov, Biomaterials Science (2019)
Glutamate Imaging Reveals Multiple Sites of Stochastic Release in the CA3 Giant Mossy Fiber Boutons. Sylvain Rama, Thomas P. Jensen, Dmitri A. Rusakov, Front. Cell. Neurosci. (2019)
A genetically encoded fluorescent sensor for in vivo imaging of GABA. Jonathan S. Marvin, Yoshiteru Shimoda, Vincent Magloire, Marco Leite, Takashi Kawashima, Thomas P. Jensen, Ilya Kolb, Erika L. Knott, Ondrej Novak, Kaspar Podgorski, Nancy J. Leidenheimer, Dmitri A. Rusakov, Misha B. Ahrens, Dimitri M. Kullmann & Loren L. Looger, Nature Methods (2019)
Monitoring intracellular nanomolar calcium using fluorescence lifetime imaging. Kaiyu Zheng, Thomas P Jensen & Dmitri A Rusakov, Nature Protocols (2018)
Monitoring single-synapse glutamate release and presynaptic calcium concentration in organised brain tissue. Jensen TP, Zheng K, Tyurikova O, Reynolds JP, Rusakov DA, Cell Calcium (2017)
Time-Resolved Imaging Reveals Heterogeneous Landscapes of Nanomolar Ca2+ in Neurons and Astroglia. Kaiyu Zheng, Lucie Bard, James P. Reynolds, Claire King, Thomas P. Jensen, Alexander V. Gourine, Dmitri A. Rusakov, Neuron (2015)
Principles of Fluorescence Spectroscopy, 3rd ed. Lakowicz, J.R., Springer (2006)
Fluorescence lifetime maesurement and biological imaging. Berezin, M.Y. & Achilefu, S., Chem. Rev. (2010)
Non-invasive imaging of skin physiology and percutaneous penetration using 5D (space, time and anisotropy) fluorescence spectral and lifetime imaging with multiphoton and confocal microscopy. Roberts, M.S., Dancik, Y., Prow, T.W., et al., Eur. J. Pharm. Biopharm. (2011)
