Optogenetics
The essence of optogenetics is introducing light-activated recombinant ion channels such as channelrhodopsin (ChR2) or halorhodopsin (NpHR) into excitable cells. Light activation of these molecules leads to an influx of ions which induces turning neurons on or off selectively. Halorhodopsin and channelrhodopsin together enable multicolor optical activation, silencing, and desynchronization of neural activity, creating a powerful neuroengineering toolbox. Photostimulation can be induced using visible or infrared light, while imaging is performed by a femtosecond IR laser. Switching between the stimulation and imaging is done at a sub-millisecond scale. Importantly the detectors are protected during the stimulation by a built-in gating system.


3D PHOTOSTIMULATION AND IMAGING
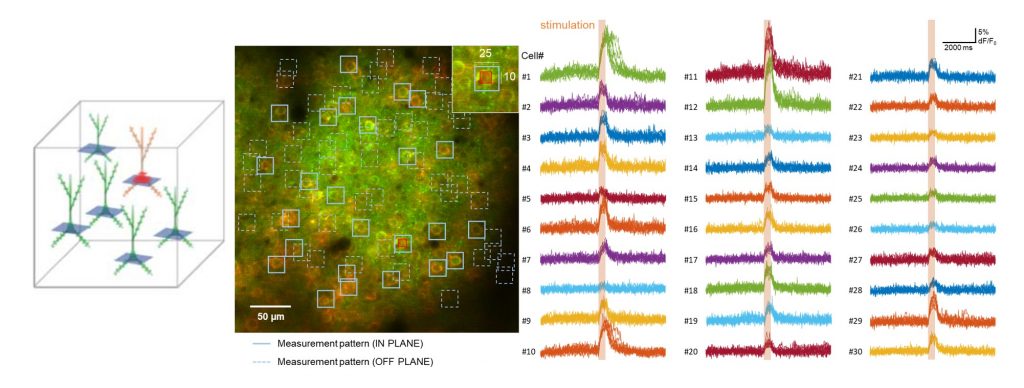
Photostimulation can be interlaced with imaging by selecting a scan area covering one or more somata situated anywhere in the 3D field of view by the FEMTO3D Atlas Dichro extension. Stimulation can be performed by the 3D chessboard scanning mode which is an advanced experimental approach, that extends 3D random-access point scanning into small local planes. Distributing the stimulation energy homogenously onto the somata of the selected cells allows maximum stimulation efficiency, thereby minimizing the possibility of photodamage to the targeted cells. The laser beam used for photostimulation excites the channelrhodopsin expressed in the cells, eliciting a controlled burst of action potentials. For calcium imaging a second scanning pattern with larger regions can be selected, enveloping cell bodies with the surrounding areas, in which the evoked activity can be followed even during sample movements. Timing of the photostimulation and the imaging can be precisely controlled by various protocols from the software GUI. This way the connectivity of the network elements can be examined, and correlated with the network function.

Full-field illumination

FEMTOSmart Resonant equipped with LED light source
The entire FOV can be stimulated with the LED source above the objective. LEDs are available at different wavelengths, exciting ChR2 at 473 nm or NpHR at 561 nm. The light source provides homogeneous illumination, and the light impulses are precisely timed and highly repeatable.
The FEMTOSmart Resonant microscope follows the changes over the whole field of view at a resolution of 31 frames per second.
Stimulation along ROIs
FEMTOSmart Galvo equipped with Multiple beam path extension
To stimulate cells or subcellular components selectively, the best solution is using the FEMTOSmart Galvo equipped with a secondary laser (Multiple Beam Path) to steer the laser beam rapidly through optimized scanning patterns such as point, line, spiral, zigzag. While scanning along points and lines, which allow stimulation on in precise locations on spines or dendrites, the spiral and zigzag patterns covering larger region enable more molecules to be stimulated simultaneously on the soma resulting activation. We offer a continuous laser tuned to 473 or 561 nm for ChR2 or NpHR activation, respectively. Precise two-photon activation of these molecules is also a viable option.
Simultaneous photoactivation and imaging
FEMTOSmart Dual equipped with Multiple beam path extension
The FEMTOSmart Dual microscope contains a galvo and a resonant scanner which function in tandem to combine the advantages of both the galvo and resonant microscopes. This is the best solution for simultaneous photostimulation and high-speed imaging.


References
Activity Patterns in the Neuropil of Striatal Cholinergic Interneurons in Freely Moving Mice Represent Their Collective Spiking Dynamics. Rotem Rehani, Yara Atamna, Lior Tiroshi, Wei-Hua Chiu, José de Jesús Aceves Buendía, Gabriela J. Martins, Gilad A. Jacobson and Joshua A. Goldberg, eNeuro, 2019
Dendrite-Specific Amplification of Weak Synaptic Input during Network Activity In Vivo. Leiron Ferrarese, Jean-Sébastien Jouhanneau, Michiel W.H. Remme, Jens Kremkow, Gergely Katona, Balázs Rózsa, Susanne Schreiber, James F.A. Poulet, Cell Reports, 2018
Topography of a Visuomotor Transformation. Thomas O. Helmbrecht, Marco dal Maschio, Joseph C. Donovan, Styliani Koutsouli, Herwig Baier, Neuron, 2018
Linking Neurons to Network Function and Behavior by Two-Photon Holographic Optogenetics and Volumetric Imaging. Dal Maschio M, Donovan JC, Helmbrecht TO, Baier H, Neuron (2017)
An optogenetic toolbox for unbiased discovery of functionally connected cells in neural circuits. Dominique Förster, Marco Dal Maschio, Eva Laurell, and Herwig Baier, Nature Communication (2017)
