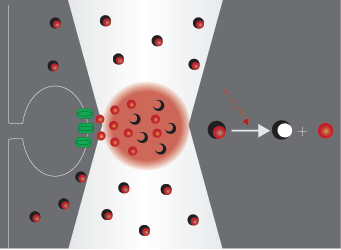
Uncaging
Uncaging means the activation of biochemically masked (‘caged’) molecules via photolysis, which mimics the physiological release of bioactive compounds. This technique is widely used in neuroscience, where the bioactive molecule is usually glutamate or another neurotransmitter. Using two-photon photostimulation with ultrafast pulsed IR laser light, very precise release of these compounds can be elicited in extremely small volumes. Two-photon imaging is a powerful opportunity to follow the changes evoked in dendrites or spines, even the distribution of receptors on the neurons can be investigated.

In vitro uncaging at well-defined points
FEMTOSmart Galvo equipped with a Multiple beam path extension
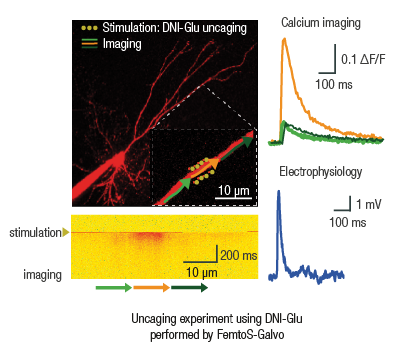
The accuracy of the excitation point, and the highly flexible scanning patterns, mean that the FEMTOSmart Galvo is the best choice for uncaging experiments. Multiple-point scanning (yellow points on the figure) is used for stimulation around spines, while multiple-line scanning (arrows) makes high-speed imaging along the dendrites possible. The secondary laser beam (Multiple beam path optional module), essential for the stimulation, is coupled to the existing light path. Thus the stimulation and the imaging can be performed near simultaneously using the galvo scanner. The microsecond-scale switching time between the stimulation and imaging is established by using of Pockels cell.
Specialized software functions for photostimulation mapping
Compounds for uncaging experiments
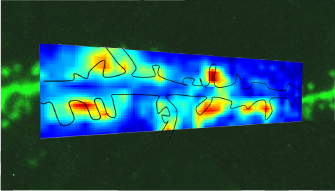
Stimulus mapping and analysis software module
This module of MES control software allows performing photostimulation mapping experiments at a range of locations and shapes. The locations can be picked manually one-by-one, along a line or in a raster, and various stimulation patterns can be selected like point, spiral, x, zigzag. It forms datasets quickly by evaluating fluorescence changes images at defined time intervals. Analysis tool for stimulation mapping can create (multichannel) map images formed by the elicited responses after a mapping experiment.
Compounds for uncaging experiments
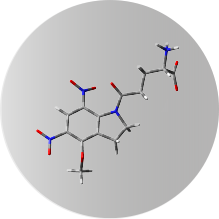
DNI-GLU-TFA synthesized by FemtoChemistry
This dinitro-indoline-masked form of glutamate releases the bioactive glutamate more rapidly than any other commercially available compound. It was developed for high-quantum yield requiring less irradiation for release, so its effective concentration is lower than other caging scaffolds. DNI-Glu is a compound developed in-house, only available from Femtonics. See also Chiovini et al., Neuron, 2014.
Do you want to learn more about all of our caged neurotransmitters?
References
Dendritic inhibition differentially regulates excitability of dentate gyrus parvalbumin-expressing interneurons and granule cells. Claudio Elgueta & Marlene Bartos, Nat. Commun (2019)
Dendritic NMDA receptors in parvalbumin neurons enable strong and stable neuronal assemblies. Jonathan H Cornford Is a corresponding author, Marion S Mercier, Marco Leite, Vincent Magloire, Michael Häusser, Dimitri M Kullmann, eLIFE (2019)
Altered dendritic spine function and integration in a mouse model of fragile X syndrome. Sam A. Booker, Aleksander P. F. Domanski, Owen R. Dando, Adam D. Jackson, John T. R. Isaac, Giles E. Hardingham, David J. A. Wyllie & Peter C. Kind, Nat. Commun. (2019)
Parsing Out the Variability of Transmission at Central Synapses Using Optical Quantal Analysis. Cary Soares, Daniel Trotter, André Longtin, Jean-Claude Béïque and Richard Naud, Front. Synaptic Neurosci. (2019)
Endocannabinoid Signaling Mediates Local Dendritic Coordination between Excitatory and Inhibitory Synapses. Hai Yin Hu, Dennis L.H. Kruijssen, Cátia P. Frias, Balázs Rózsa, Casper C. Hoogenraad, Cell Reports (2019)
A compact holographic projector module for high-resolution 3D multi-site two-photon photostimulation. Mary Ann Go, Max Mueller, Michael Lawrence Castañares, Veronica Egger, Vincent R. Daria, PLOS One (2019)
Coincidence Detection within the Excitable Rat Olfactory Bulb Granule Cell Spines. S. Sara Aghvami, Max Müller, Babak N. Araabi and Veronica Egger, J. Neurosci. (2019)
Disentangling astroglial physiology with a realistic cell model in silico. Leonid P. Savtchenko, Lucie Bard, Thomas P. Jensen, James P. Reynolds, Igor Kraev, Nikolay Medvedev, Michael G. Stewart, Christian Henneberger & Dmitri A. Rusakov, Nat. Commun. (2018)
Voltage Gated Calcium Channel Activation by Backpropagating Action Potentials Downregulates NMDAR Function. Anne-Kathrin Theis, Balázs Rózsa, Gergely Katona, Dietmar Schmitz and Friedrich W. Johenning, Front. Cell. Neurosci. (2018)
High efficiency two-photon uncaging coupled by the correction of spontaneous hydrolysis. Dénes Pálfi, Balázs Chiovini, Gergely Szalay, Attila Kaszás, Gergely F. Turi, Gergely Katona, Péter Ábrányi-Balogh, Milán Szőri, Attila Potor, Orsolya Frigyesi, Csilla Lukácsné Haveland, Zoltán Szadai, Miklós Madarász, Anikó Vasanits-Zsigrai, Ibolya Molnár-Perl, Béla Viskolcz, Imre G. Csizmadia, Zoltán Mucsi and Balázs Rózsa, Org. Biomol. Chem. (2018)
Super-Resolution Imaging of the Extracellular Space in Living Brain Tissue. Jan Tønnesen, V.V.G. Krishna Inavalli, U. Valentin Nägerl, Cell (2018)
Metaplasticity at CA1 Synapses by Homeostatic Control of Presynaptic Release Dynamics. Cary Soares, Kevin F.H. Lee, Jean-Claude Béïque, Cell Reports (2017)
Imaging membrane potential changes from dendritic spines using computer-generated holography. Dimitrii Tanese, Ju-Yun Weng, Valeria Zampini, Vincent de-Sars, Marco Canepari, Balazs J. Rozsa, Valentina Emiliani, Dejan Zecevic, Neurophotonics (2017)
Cell-type–specific inhibition of the dendritic plateau potential in striatal spiny projection neurons. Kai Du, Yu-Wei Wu, Robert Lindroos, Yu Liu, Balázs Rózsa, Gergely Katona, Jun B. Ding, and Jeanette Hellgren Kotaleski, PNAS (2017)
Correlated Synaptic Inputs Drive Dendritic Calcium Amplification and Cooperative Plasticity during Clustered Synapse Development. Kevin F.H. Lee, Cary Soares, Jean-Philippe Thivierge, Jean-Claude Béïque, Neuron (2016)
Electrical behaviour of dendritic spines as revealed by voltage imaging. Marko A. Popovic, Nicholas Carnevale, Balazs Rozsa; Dejan Zecevic, Nature Communications (2015)
Local Postsynaptic Voltage-Gated Sodium Channel Activation in Dendritic Spines of Olfactory Bulb Granule Cells. Wolfgang G. Bywalez, Dinu Patirniche, Vanessa Rupprecht, Martin Stemmler, Andreas;V.M. Herz, Denes Palfi, Balazs Rozsa, Veronica Egger, Neuron (2015)
Quantitation of various indolinyl caged glutamates as their o-phthalaldehyde derivatives by high performance liquid chromatography coupled with tandem spectroscopic detections: derivatization, stoichiometry and stability studies. Vasanits-Zsigrai A, Majercsik O, Toth G, Csampai A, Haveland-Lukacs C, Palfi D, Szadai Z, Rozsa B, Molnar-Perl I, J Chromatogr A. (2015)
Molecular Tattoo: Subcellular Confinement of Drug Effects. Miklos Kepiro, Boglarka H. Varkuti, Anna A. Rauscher, Miklos S.Z. Kellermayer, Mate Varga, Andras Malnasi-Csizmadia, CellPress (2015)
Palmitoylation of LIM Kinase-1 ensures spine-specific actin polymerization and morphological plasticity. Joju George, Cary Soares, Audrey Montersino, Jean-Claude Beique, Gareth M Thomas, eLIFE (2015)
Combined two-photon imaging, electrophysiological, and anatomical investigation of the human neocortex in vitro. Balint Peter Kerekes, Kinga Toth, Attila Kaszas, Balazs Chiovini, Zoltan Szadai, Gergely Szalay, Denes Palfi, Attila Bago, Klaudia Spitzer, Balazs Rozsa, Istvan Ulbert, Lucia Wittner, Neurophotonics (2014)
Spine neck plasticity regulates compartmentalization of synapses. J Tønnesen, G Katona, B Rozsa, U V Nägerl, Nature Neuroscience (2014)
Dendritic spikes induce ripples in parvalbumin interneurons during hippocampal sharp waves. B Chiovini, G F Turi, G Katona, A Kaszas, D Palfi, P Maak, G Szalay, M F Szabo, Z Szadai, Sz Kali and B Rozsa, Neuron (2014)
The kinetics of multibranch integration on the dendritic arbor of CA1 pyramidal neurons. Sunggu Yang, Valentina Emiliani and Cha-Min Tang, Front. Cell. Neurosci. (2014)
Differential Subcellular Targeting of Glutamate Receptor Subtypes during Homeostatic Synaptic Plasticity. Cary Soares, Kevin F. H. Lee, Wissam Nassrallah and Jean-Claude Béïque, J. Neurosci. (2013)
GnRH Neurons Elaborate a Long-Range Projection with Shared Axonal and Dendritic Functions. Michel K. Herde, Karl J. Iremonger, Stephanie Constantin and Allan E. Herbison, J. Neurosci. (2013)
Dendritic Hold and Read: A Gated Mechanism for Short Term Information Storage and Retrieval. Mariton D. Santos, Michael H. Mohammadi, Sunggu Yang, Conrad W. Liang, Joseph P. Y. Kao, Bradley E. Alger, Scott M. Thompson, Cha-Min Tang PLOS One (2012)
Parallel optical control of spatiotemporal neuronal spike activity using high-speed digital light processing. Jason Jerome, Robert C. Foehring, William E. Armstrong, William J. Spain and Detlef H. Heck, Front. Syst. Neurosci. (2011)
Roller Coaster Scanning reveals spontaneous triggering of dendritic spikes in CA1 interneurons. G. Katona, A. Kaszas, G. F. Turi, N. Hajos, G. Tamas, E.S. Vizi, B. Rozsa, PNAS (2011)
