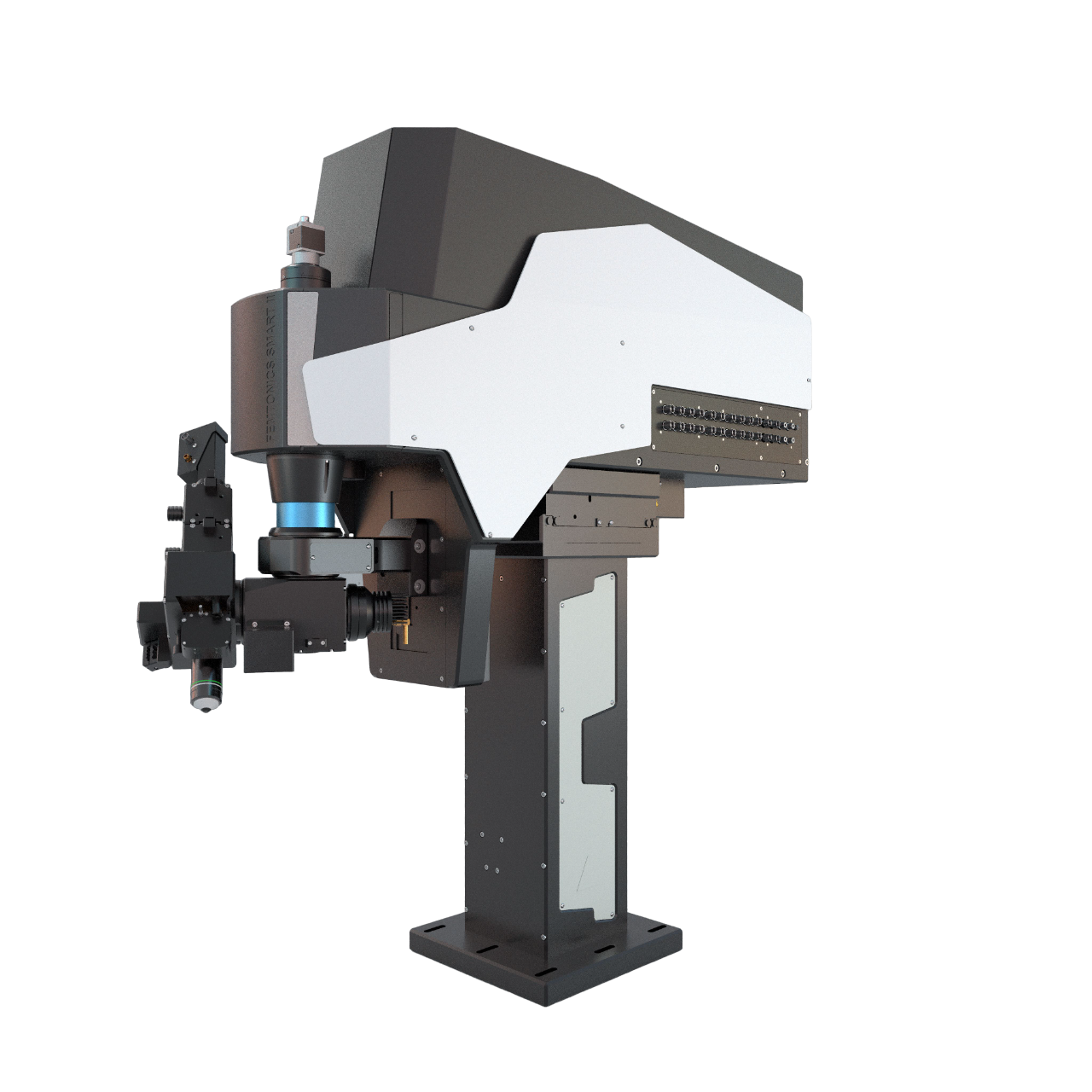

The FEMTOSmart series consists of fully customizable two-photon microscopes, fitting the customers’ needs perfectly. The special feature of these devices is the elevated body, which can move in X, Y, and Z directions, providing ample room under the objective for optimal sample positioning.
This feature makes them suitable for performing in vivo experiments, such as in the brain, in a wide range of model organisms, from zebrafish larvae, through mice navigating in virtual reality to non-human primates.
The FEMTOSmart series, with its three members, represents traditional two-photon imaging systems capable of galvo and resonant scanner-based functional imaging.
Watch our webinar about the FEMTOSmart multiphoton microscope.
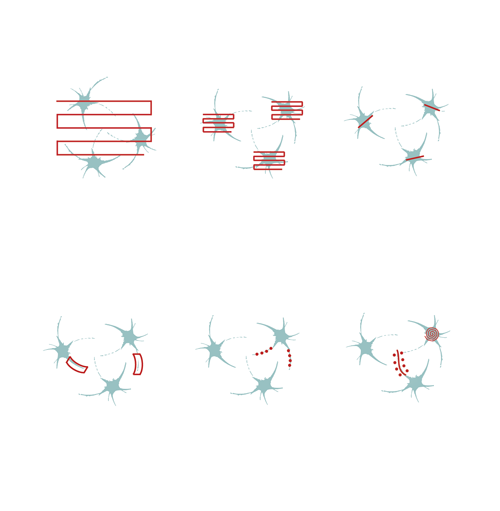
Flexible scanning patterns, such as 2D random-access point, 2D multiple-line, and folded-frame scanning supports the manual selection of individual cells, of the brain or other organs, in a 2D plane. By skipping the measurement of the entire field, it is possible to maintain a high scanning speed and signal-to-noise ratio. Fast-frame scanning and its combination with fast Z-focusing (Piezo objective positioner) is a well-known approach for performing calcium imaging or other practical applications in neuronal networks in two or three dimensions.
By using flexible 2D scanning methods (e.g. multiple line scanning), you can follow the tortuous protrusions of the dendritic arbors of neurons in a fast and precise way. The microscopes equipped with Piezo objective positioner enable you to follow the activity of three-dimensional arborizations of dendrites, using calcium imaging or other useful applications and provide cost-effective, alternative solutions compared to the acousto-optic scanner-based scanning methods.
Three-photon (3P) microscopy allows noninvasive functional imaging by making cells visible in deep tissues, with a high spatial resolution and better contrast compared to two-photon excitation. Longer excitation wavelengths come with less scattering in biological tissues, which makes extending the penetration depth feasible, reduces out-of-focus excitation, and increases the signal-to-noise ratio. For 3P imaging, equip our FEMTOSmart microscopes with the 3P range microscopy optional module.
To stimulate cells or subcellular components, you can steer the laser beam rapidly through the entire FOV using a LED light source, or in optimized scanning patterns that can take the shape of a point, a line, a spiral, or zigzag using Multiple beam paths. While scanning at points or along lines allows for stimulation at precise locations, such as spines or dendrites, the spiral and zigzag patterns covering larger regions enable the simultaneous stimulation of multiple molecules on the neuronal soma, resulting in activation. Performing photostimulation along with calcium imaging allows us to follow the evoked changes in dendrites, spines, or even the receptor distribution on neurons.
The galvo scanner combined with our intelligent control software enables the user to select numerous scanning patterns covering widely distributed ROIs. Multiple frame scanning allows focusing on cell bodies, multiple line scanning enables us to follow action potentials along dendrites, random-access point scanning allows for the measurement or simultaneous photostimulation and calcium imaging in subcellular components with the highest temporal resolution. Our software features (real-time display, analysis functions, ∆F/F calculations, the integrated parallel data acquisition of electrical recordings) aid the understanding of physiological processes in your research focus.
Multiple line scanning has been developed for researchers aiming to resolve dendritic and even spine activity of neurons with a near real-time measurement mode. During this scanning mode, the X and Y mirrors direct the laser beam in a flexible way along straight lines or complex curves. The scanner spends most of its time collecting signals from these lines, skipping intermediate sections between them. Therefore, the scanning speed and the signal-to-noise ratio (SNR) of the signals from the multi-site ROIs increases 3- to 4-fold compared to frame scanning.
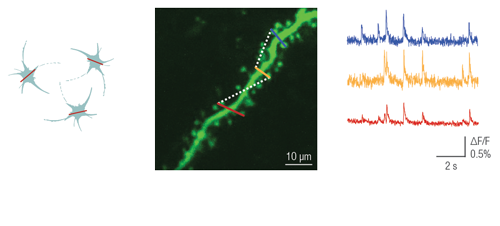
This patented method enables users to image a confined area along a line, where the shape of the selected regions can be straight or curved. This advanced scanning method is useful for imaging single cell bodies in different regions of the specimen or performing calcium imaging along winding dendrites of neurons, with their protrusions, even in the brain of moving animals.
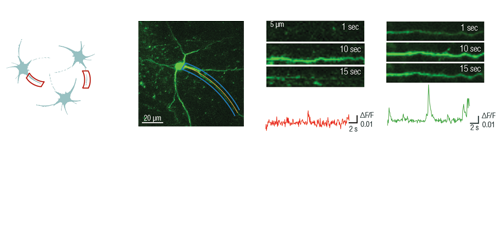
Optogenetics, uncaging, and other photostimulation techniques are also supported by our unique scanning patterns and their combinations. Random-access point scanning can be used for stimulation in femtoliter volumes of the brain, near dendritic spines of neurons, where the duration of the stimulation can be set from microseconds to seconds. The evoked signals can be followed by calcium imaging along the dendrite using line scanning near-simultaneously with photostimulation.
The FEMTOSmart Galvo microscope is controlled by and works together with our modular measurement control and analysis software package called MES. MES is written in a MATLAB environment, enabling rapid code development, and makes the data accessible to the users. Its design was inspired by the daily laboratory experiences of researchers in the field of cellular and network imaging. MES supports 2D ROI selection and immediate ROI activity analysis, which are necessary for high throughput measurements when following neuronal activity, e.g., performing calcium imaging.
In the FEMTOSmart Resonant microscope, Femtonics combines high-speed and high-sensitivity imaging of living tissues by using a fast resonant scanner. Resonant-scanner-based raster scanning acquires images at a 31 frames per second speed for hours, which is ~5 times faster than galvanometer-based scanning in the entire field of view. The velocity of the resonant scanner is non-linear: the speed is different in the center and at the edges of the frame. In the microscope, Pockels cell limits the scanning range to the portion, where scanning velocity is near-linear, avoiding photobleaching/photodamage at the two sides of the image. Scan electronics perform dynamic pixel dwelling for data linearization, and to cancel out image distortion. This Resonant-scanner-based member of the FEMTOSmart family is perfectly suitable for following neuronal activity in the brain, e.g., performing calcium imaging, or other practical applications with a high speed, even for long periods.
![]()
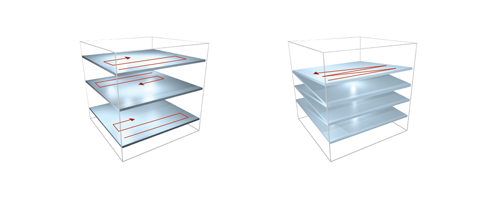
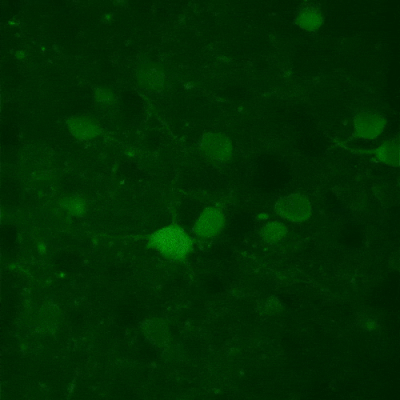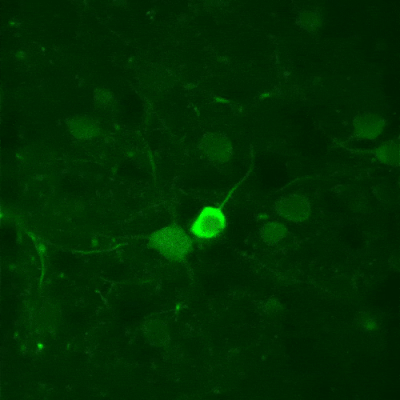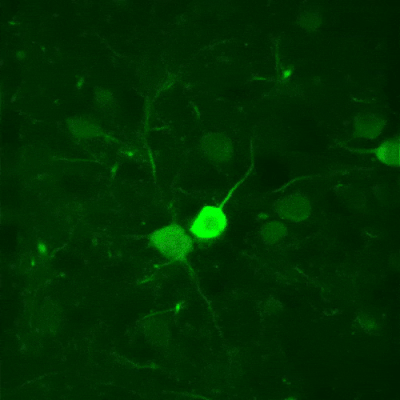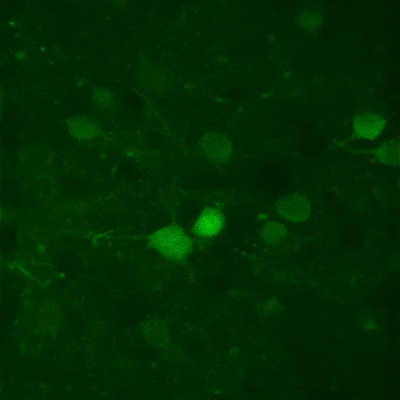
Fast XY-scanning, combined with a fast movement in the Z plane, ensures the near real-time measurement of a volume in 3D, which enables us to study activity changes in 3D cellular networks or the morphology of the brain or other organs. Movement in the Z plane can be performed by a Piezo objective positioner, and two scanning methods are available: frame by frame scanning and n-frame ramping.
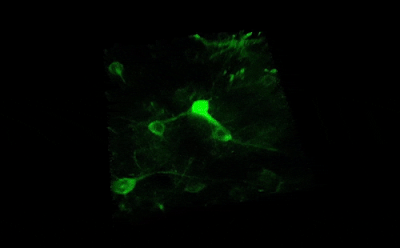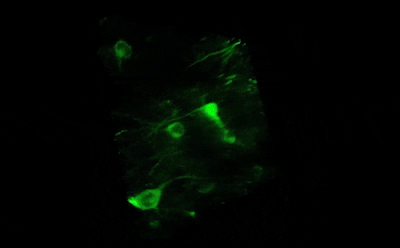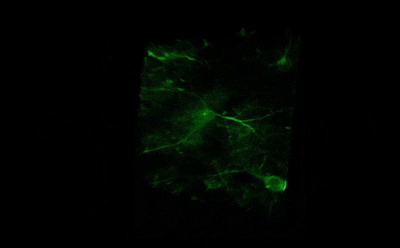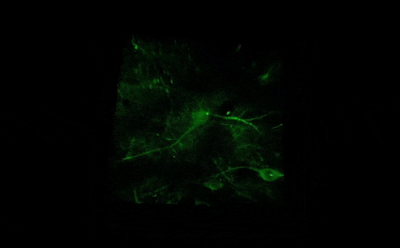
Tile imaging was developed to enable the imaging of larger specimens in their entirety. The field of view can be easily magnified by assembling single images to form one large super-image of the sample of interest. Tile imaging is applicable for large field-of-view imaging, calcium imaging, super-resolution imaging and can provide a solution for capturing anatomical images automatically in a high resolution. The pictured image exhibits 3 x 4 frames (690×690 µm) of the cells of a Tilia stem fitted into a single 1800×2370 µm image. The image was made using the Tile Scan plugin integrated into the control software by MEScAPI. For more information please contact our specialists.
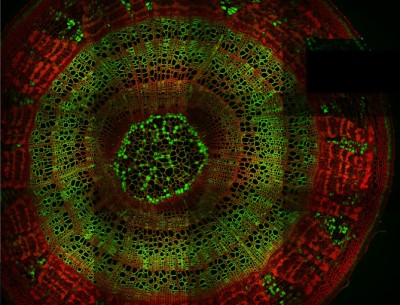
Cells distributed in one or more layers can be stimulated and imaged with high speed using the Resonant microscope equipped with precisely timed LED light sources. LEDs are available at different wavelengths, exciting ChR2 at 473 nm or NpHR at 561 nm, which makes the microscope suitable for performing optogenetic experiments. The microsecond-scale switching time between stimulation and imaging is achieved by using a Pockels cell and gated detectors.

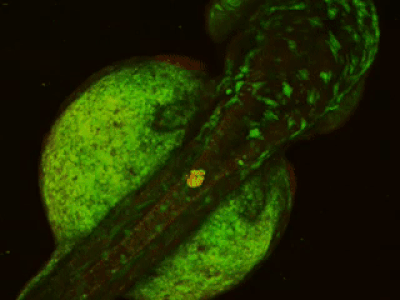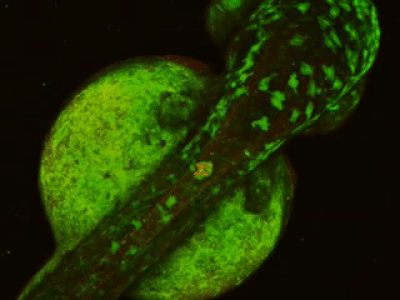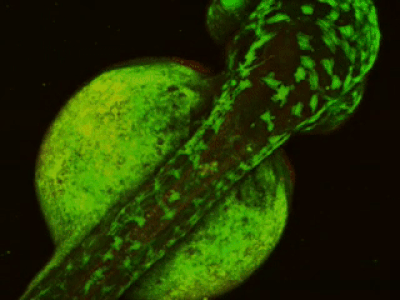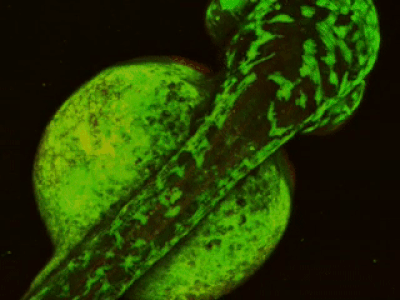
While two-photon excitation allows for in-depth imaging and a fine spatial resolution, the high frame scanning rate of the resonant scanner provides a high temporal resolution. This feature means that the microscope is suitable for measuring rapid events in living cells, neural networks, or other circuits. A high frame scanning rate and unlimited video streaming combined with the possibility of performing automated measurements support long-term studies, such as following learning processes, memory retrieval, associative learning, development of model organisms, etc. The middle figure shows an early stage of development of a zebrafish embryo, whose ontogenesis was followed and recorded for over a day, from a low cell state.
The FEMTOSmart Resonant is driven by the MESc measurement control and data analysis software. MESc is under active development for performing imaging in cells and neuronal networks, such as calcium imaging, supporting more and more features and the Femtonics hardware. MESc supports fast Z-stack recording, unlimited measurement time, and unlimited video streaming to the hard drive, which are necessary for long-time measurements in a volume.
The FEMTOSmart Dual microscope contains both galvanometric and resonant scanners, providing all advantages of the two imaging techniques. Using the two scanner functions in tandem is a perfect solution for photostimulation in the brain. The galvanometric scanner directs the laser to cells or subcellular components selectively. Selectivity is ensured by scanning along arbitrary line patterns, placed on cell bodies or dendritic segments. With the resonant scanner, you can follow the changes in the cells of a neural network with calcium imaging, collecting imaging data simultaneously by high-speed frame scanning of the surrounding area. The timing of photostimulation and detector gating is controlled by protocols designed on the user-friendly GUI. The Tilting objective, as an optional module, can increase the accessibility of the objective to the sample.
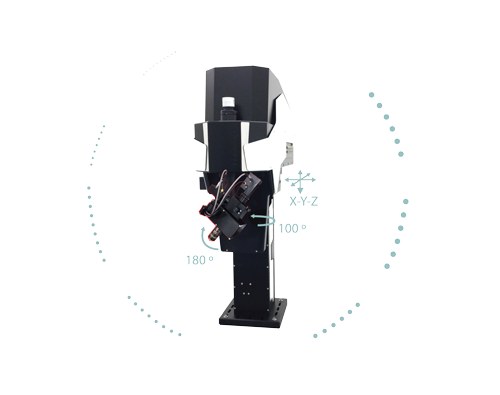
The column-based X-Y-Z moving body of the FEMTOSmart contains the scanner unit, the control circuits, and an internal light path. The elevated body enables you to place any kind of moving table or virtual reality under the objective. The accessibility of the sample is ensured by two motors: the objective can move in a 50 mm range in the Z direction, and the microscope body can also move in a 50 mm range in the XY directions around the central position, due to the shifting base.
The fine-tuned optical construction allows imaging to a depth of 850 µm, and a wavelength range from the visible to the infrared range, allowing even 3P excitation. The scanners provide the highest resolution currently available. Detection is performed by our patented travelling detector system, where the highest quality GaAsP PMTs and associated optical elements are mounted on the objective arm, as close as possible to the objective, which helps to keep the photon collection ratio high.
The microscope’s modular nature allows us to assemble the components, and further upgrade the system to fit the customer’s needs perfectly. Multiple lasers can be coupled into the microscope, either for imaging or photoactivation. The system can be equipped with a lot of optional modules, enabling it to be adapted to a wide range of biological applications, from calcium imaging to optogenetics, uncaging and dendritic imaging.
We are proud to have installed our state-of-the-art multiphoton microscopes in prestigious laboratories from all around the world: our users are excellent scientists in their fields. Studies made possible by our systems are being published in high-impact journals. In dedicated Demo Rooms, you can even become acquainted with these systems in their natural habitat. Browse the locations of these labs below, and do not hesitate to contact us if you would like a demonstration organized!
We would like to call your attention to our CENTER OF EXCELLENCE program as well. Besides functioning as Demo Rooms themselves, laboratories and institutes participating in this program will benefit from our constant technological and scientific support and our latest high-tech developments. If you are interested in starting such a fruitful partnership, please, contact our team – we are excited to hear from you.
Budapest, Hungary

Group Leader: Dr. Ádám Dénes
Institute of Experimental Medicine
Eötvös Loránd Research Network
London, United Kingdom

Group Leader: Prof. Dmitri Rusakov
Laboratory of Synaptic Imaging
School of Life & Medical Sciences – Faculty of Brain Sciences – Institute of Neurology
University College London (UCL)
Bordeaux, France

Group Leader: Dr. Andreas Frick
Institut François Magendie
Bordeaux Neurocampus
Marseilles, France

Group Leader: Dr. Ivo Vanzetta
Physiology & Physiopathology of Neuronal Networks Group
French National Institute of Health and Medical Research
Taipei, Taiwan

Principal Investigator: Prof. Yu-Wei Wu
IMB Academia Sinica
Trodheim, Norway

Group Leader: Nobel laureate Prof. May-Britt Moser
Kavli Institute for Systems Neuroscience
NTNU – Norwegian University of Science and Technology
Basel, Switzerland
Head of Research Group: Prof. Dr. Flavio Donato
Development of neuronal circuits and cognitive functions
Biozentrum, University of Basel
Tübingen, Germany

Head of Department: Prof. Dr. Olga Garaschuk
Contact researcher: Dr. Yury Kovalchuk
Institute of Physiology
University of Tübingen
Regensburg, Germany

Group Leader: Prof. Dr. Veronica Egger
University of Regensburg
Berlin, Germany

Group Leader: Dr. David Owald
Institute of Neurophysiology , Charité – Universitätsmedizin Berlin
Munich, Germany

Group Leader: Prof. Dr. med. Nikolaus Plesnila
Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich
Modena, Italy

Group Leader: Prof. Albertino Bigiani
Contact Researcher: Prof. Jonathan Mapelli
Experimental and Computational Neurophysiology Research Group
Università degli Studi di Modena e Reggio Emilia