Voltage Imaging
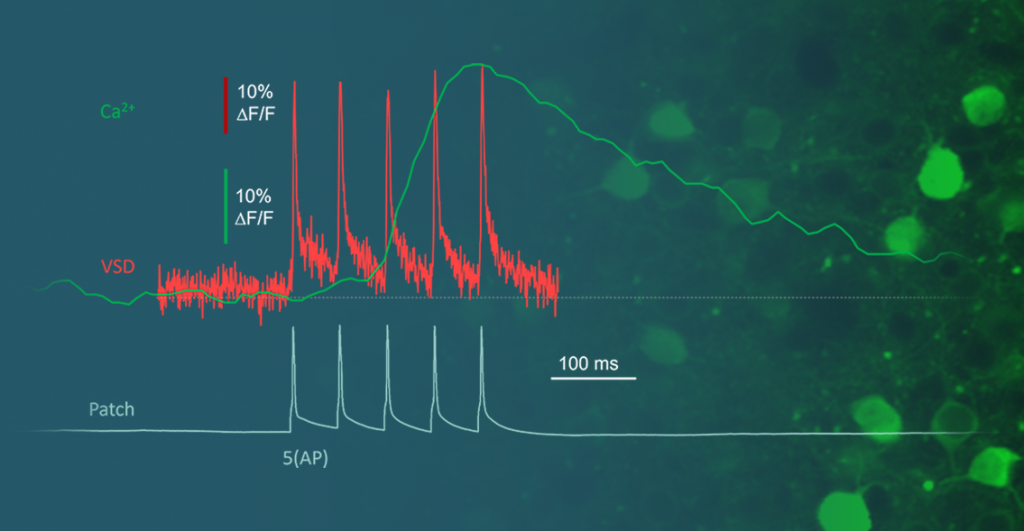
Genetically encoded voltage indicators (ASAP, JEDI2P, etc.) have recently attained a level of quality that renders them valuable tools for studying membrane potentials throughout cells. However, a significant challenge has persisted: the lack of sufficiently fast equipment to capture their dynamics. Until now, there has been a shortage of systems capable of imaging at speeds exceeding kHz, approaching the electrophysiological standard of 10 kHz. The Femtonics 3D AO Atlas addresses this gap by enabling high-speed, three-dimensional voltage imaging. Equipped with the real-time motion correction module, the 3D Real-Time Motion Correction, it achieves rapid imaging without motion artifacts and ensures high signal-to-noise ratio (SNR) for precise voltage imaging. Read more
Network Imaging
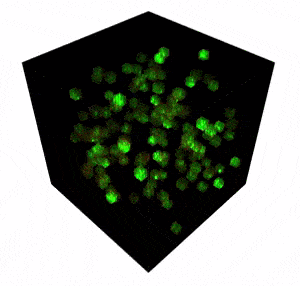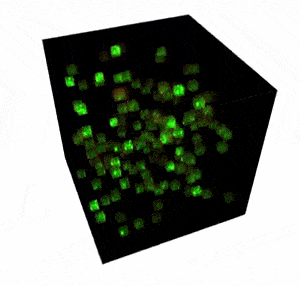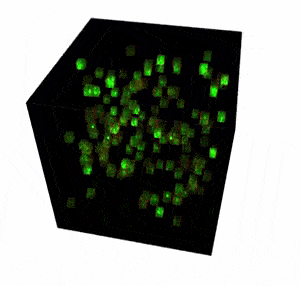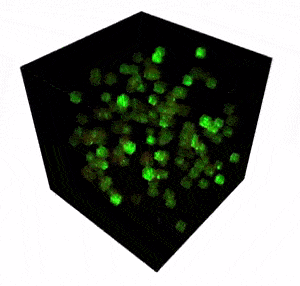
All sensations and behaviors are encoded in dynamic activity patterns of neural networks. In other words, complex structures of many individual neurons responding to environmental features, visual or auditory stimuli,, reward or punishment, etc. These neural systems extend over 3D space and, in most cases, cross many cortical layers of the brain. With two-photon microscopy enabling neuroscientists to reach the deep regions of the brain (down to 850 µm), it is now possible to study neural networks at a high spatiotemporal resolution. Read more
Dendritic Imaging
Dendrites and dendritic spines are made of fine, thin and elongated segments therefore they are difficult to study. However, using two-photon technology, we are able to collect signals from deeper regions of the brain, while avoiding phototoxicity. Additionally, spatially confined, regional scanning of axons, dendrites and spines allows detecting even subthreshold signals with high signal-to-noise ratio (SNR). We have several configurations aiding to visualize dendritic arborization, and perform functional measurements in vivo and in vitro. Now, with the help of our new real time motion-correction called Femtonics 3D Real-Time Motion Correction motion artifacts even in vivo in dendritic measurements will no longer be a problem! Read more
Behavioral Studies
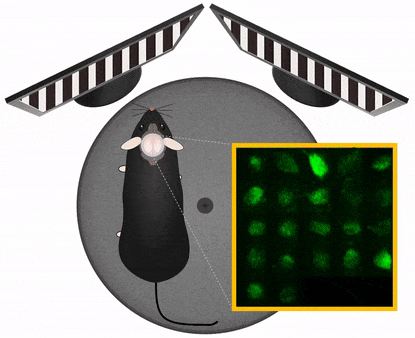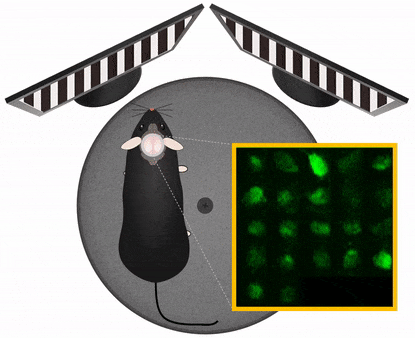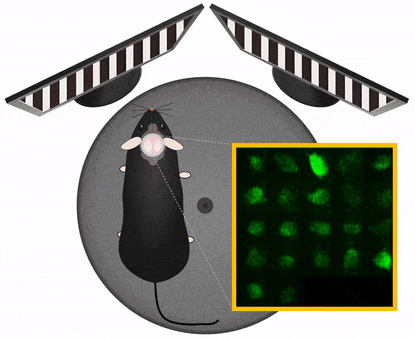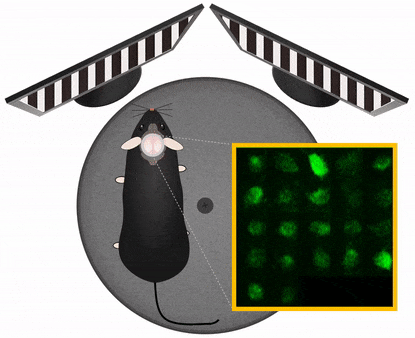
The Central Nervous System organizes behavior, therefore to study CNS functions works best in a behavioral context. In this context it is important to manipulate experimental variables in a precisely controlled way. Those variables may be stimulus information, task instructions, reward or aversive outcome, all affecting the related pattern of brain activation. Monitoring and manipulating behavior together with imaging neural network activity in the awake behaving animal is essential to establish the relation between the cellular/network and behavioral level. Read more
3P Imaging
Three-photon (3P) microscopy allows noninvasive structural and functional imaging by making cells visible in deep tissues with high spatial resolution and better contrast compared to two-photon excitation. The optical design required for 3P excitation establishes higher axial resolution than that of two-photon microscopy. The spectral window enables three-photon excitation of a variety of fluorophores, such as the current generations of protein-based genetically encoded calcium indicators (e.g. GCaMP6) and the repetition rate of the laser source is adequate for imaging Ca2+ transients produced from neural activity.
The video shows an approximately 1000 μm thick 3P z-stack from a mouse cortex. Purple: blood vessels, green: GFP labelled pericytes. Footage courtesy of Dr. Severin Filser. Read more
FLIM
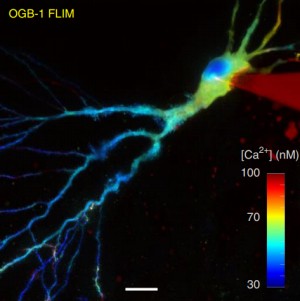
Fluorescence-lifetime imaging microscopy – FLIM
Fluorescence-lifetime imaging microscopy (FLIM) delivers both structural (intensity image) and functional (lifetime image) information and minimizes the imaging effect of photon scattering in thick layers of sample. FLIM provides an accurately quantitative assessment of changes of many biophysical parameters in a target molecular microenvironment, without being affected by the intensity of the emission, quenching or uneven fluorophore distribution. The most frequent FLIM applications are the measurement of molecular environment parameters, molecular interaction and distance measurements by Förster resonance energy transfer (FRET), besides studying the metabolic state of cells and tissues via their autofluorescence. Two-photon fluorescence-lifetime imaging microscopy (2P-FLIM) meets the needs of neuroscientific research because of the combined advantages of two-photon excitation and fluorescence-lifetime imaging: superior depth penetration and decreased photo-bleaching, owing to long excitation wavelengths, and inherent optical sectioning. Read more
Blood Flow
Following blood flow deep in the living tissue
Abnormalities in hemodynamics are linked to various disease states, such as stroke, Alzheimer’s, etc., however, to better understand these associations, more precise optical techniques are needed for interrogating blood flow velocity in vivo in 3D. Acousto-optic (AO) multiphoton microscopy is a superior technique to perform blood flow tracking at the cellular level, at a high spatiotemporal resolution deep in the living 3D tissue. The unique scanning modes of the FEMTO3D Atlas allow you to record continuously along intricate 3D patterns, and its extensions make it possible to follow multiple parameters at the same time, like blood flow and cellular activity. These applications can be useful in various fields, such as neuroscience, immunology, dermatology, oncology and more. Read more
Optogenetics

The essence of optogenetics is introducing light-activated recombinant ion channels such as channelrhodopsin (ChR2) or halorhodopsin (NpHR) into excitable cells. Light activation of these molecules leads to an influx of ions which induces turning neurons on or off selectively. Halorhodopsin and channelrhodopsin together enable multicolor optical activation, silencing, and desynchronization of neural activity, creating a powerful neuroengineering toolbox. Photostimulation can be induced using visible or infrared light, while imaging is performed by a femtosecond IR laser. Switching between the stimulation and imaging is done at a sub-millisecond scale. Importantly the detectors are protected during the stimulation by a built-in gating system. Read more
Uncaging
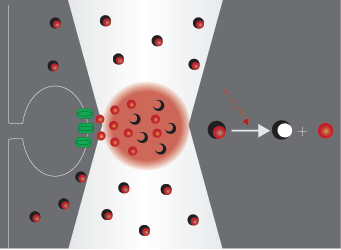
Uncaging means the activation of biochemically masked (‘caged’) molecules via photolysis, which mimics the physiological release of bioactive compounds. This technique is widely used in neuroscience, where the bioactive molecule is usually glutamate or another neurotransmitter. Using two-photon photostimulation with ultrafast pulsed IR laser light, very precise release of these compounds can be elicited in extremely small volumes. Two-photon imaging is a powerful opportunity to follow the changes evoked in dendrites or spines, even the distribution of receptors on the neurons can be investigated. Read more
Electrophysiology
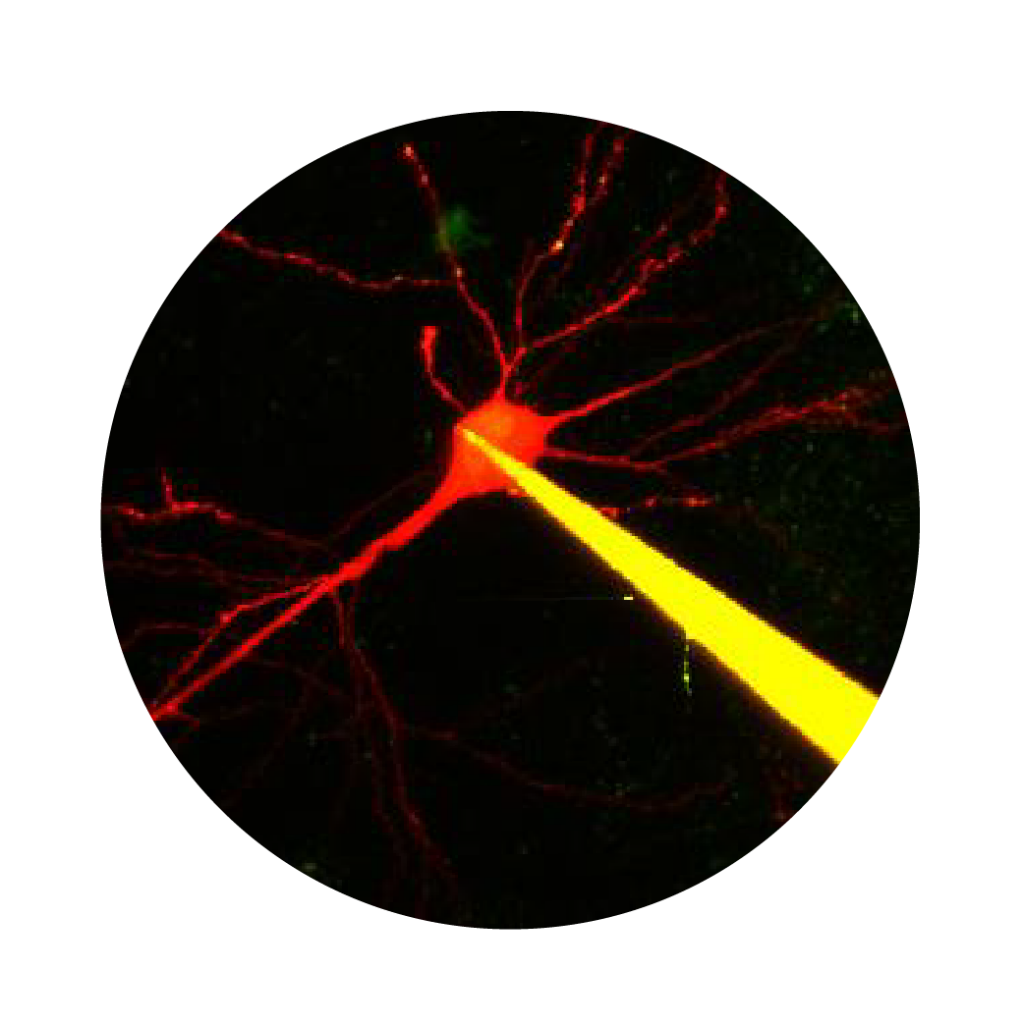
Two-photon Ca2+ imaging and electrophysiological data acquisition provide excellent tools for the study of neural cell and network activity. Using the two methods simultaneously, it is possible to follow potential fluctuations and relate them to changes in their Ca2+ levels. While the two-photon Ca2+ imaging technique shows events in multiple small areas (distinguishable parts of dendrites and spines) at high spatial resolution (less than 1 µm) but low temporal resolution (>100 ms), electrophysiology can cover changes over larger areas (cells or extensive areas) at low spatial resolution (~150 µm), and the changes can be followed considerably faster (<1 ms). Read more
Deep Functional Imaging
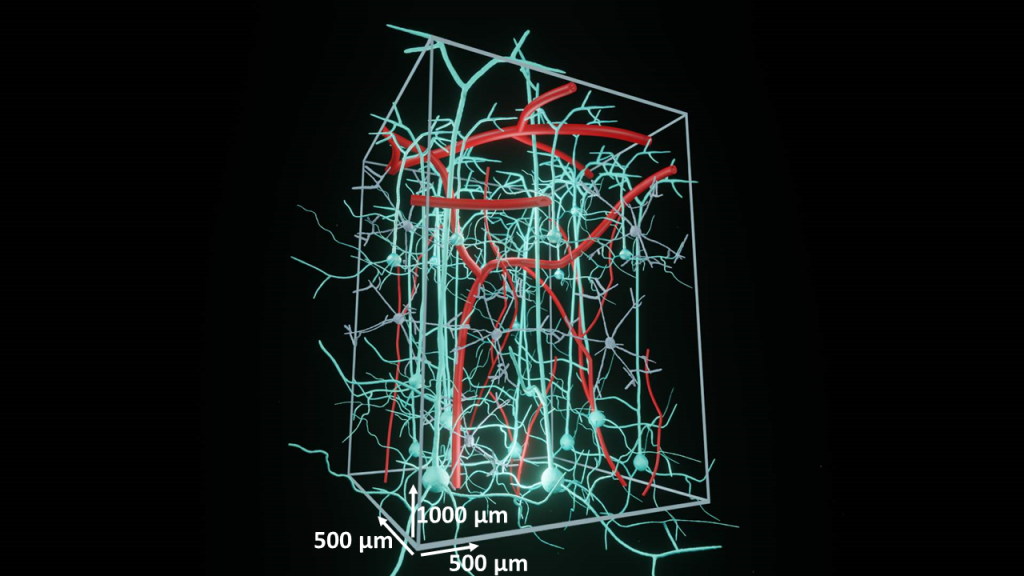
One of the most significant advantages of two-photon microscopy is the ability to examine not only the tissue’s surface but also its deeper layers. While confocal microscopy can penetrate the tissue, it is limited to just a few dozen micrometers.
Thus, a fundamental question arises: How deep can we effectively visualize within the brain?
The answer depends on a multitude of external parameters, including the laser’s wavelength and type, quality of labeling, tissue type, and preparation quality, among others. Furthermore, the imaging solutions themselves play a pivotal role in achieving depths surpassing even 1mm from the surface. In this context, we present our approach to Deep Functional Imaging. Read more
