MOUSE VIRTUAL REALITY SYSTEM – VR
Unlock Unprecedented Research Capabilities
Voltage Imaging
Genetically encoded voltage indicators (ASAP, JEDI2P, etc.) have recently attained a level of quality that renders them valuable tools for studying membrane potentials throughout cells. However, a significant challenge has persisted: the lack of sufficiently fast equipment to capture their dynamics. Until now, there has been a shortage of systems capable of imaging at speeds exceeding kHz, approaching the electrophysiological standard of 10 kHz. The Femtonics 3D AO Atlas addresses this gap by enabling high-speed, three-dimensional voltage imaging. Equipped with the real-time motion correction module, the FocusPinner, it achieves rapid imaging without motion artifacts and ensures high signal-to-noise ratio (SNR) for precise voltage imaging.
Indicators:
The rapidly evolving voltage sensor technology is going to determine the next decade of neuroscience. Voltage signals have faster kinetics and thus provide and require superior temporal resolution. The sensors report individual action potentials even within fast bursts. Conventional imaging techniques are not fast enough to capture these transients.
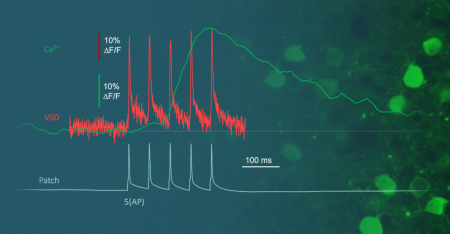
Figure 1: Visualizing the speed of the signal and the proof of necessity for
Acousto-Optical scanning technology
. A) Green: Ca2+ from a PV interneuron sampled at 30 Hz. Red: JEDI2p sampled at 8.4 kHz. Blue: Patch clamp electrophysiological recording of 5 evoked Action Potentials sampled at 20 kHz.
B) Same measurement down-sampled to 80, 40, and 21 Hz.
1. In vivo voltage imaging with FEMTO3D Atlas
One of today’s most interesting and challenging task is to measure voltage signals from neurons while the model animal is active. Even recently published in vivo voltage imaging studies have several limiting factors. Femtonics has overcome these limitations and allowed researchers to have higher success rate, higher SNR, higher number of recording locations in 3D, better focus on the dendrites and alternative ways to investigate and interact with neuronal population. All measurement techniques can be easily applied in head-fixed behaving animals too, due to the 3D Real-Time Motion Correction, which enables the high SNR in vivo as well.
Attila Losonczy, MD, PhD – Professor of Neuroscience; Principal Investigator at Columbia’s Zuckerman Institute
- Increased SNR measurements due to 3D Real-Time Motion Correction
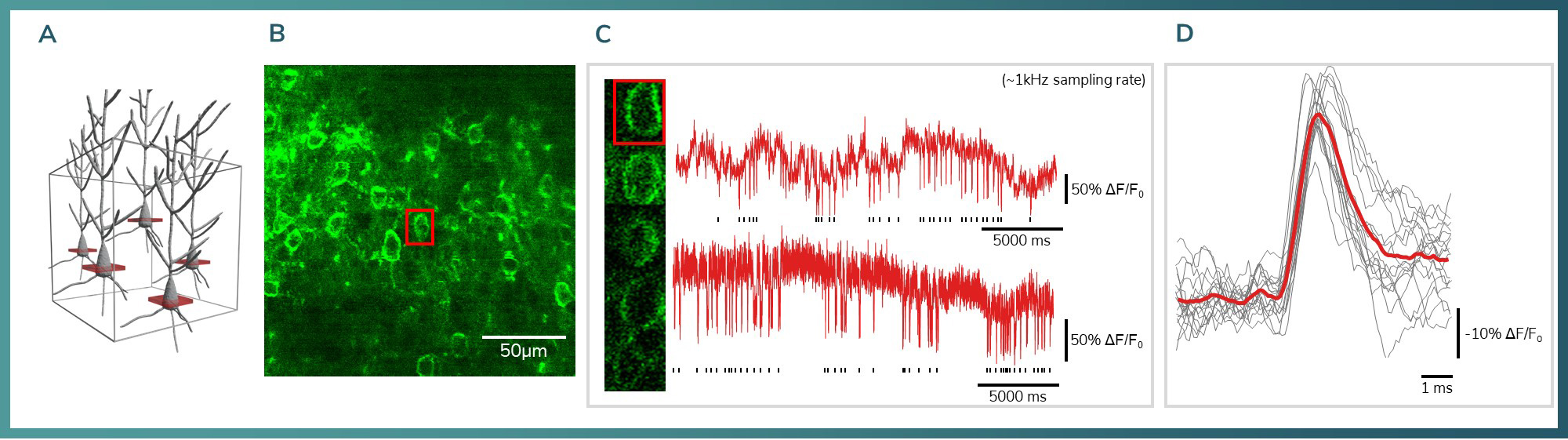
Figure 2: A) 3D model showing the relative locations of the measured neurons. B) Quality JEDI2P labelling in the visual cortex L2/3. C) Example traces showing the SNR with 1 kHz sampling from a behaving animal. Bottom: Chessboard scanning of 5 individual neurons from different Z planes. D) Average of individual APs. (n=17)
- Increased number of somatic recordings
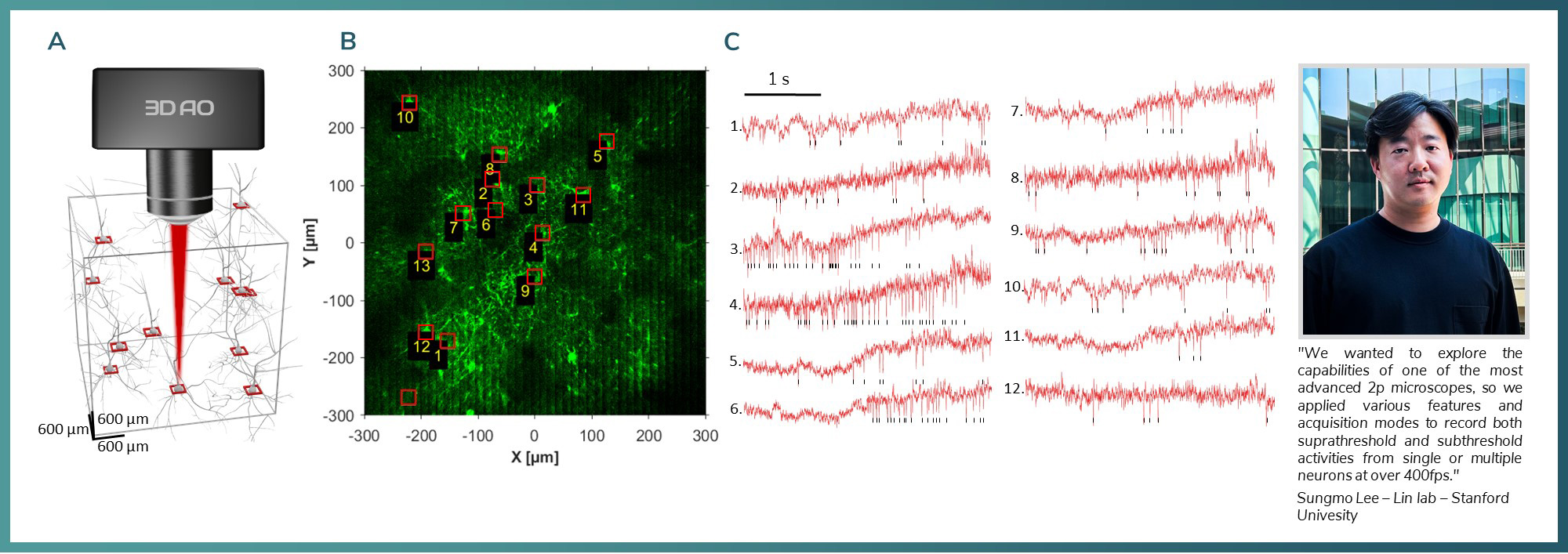
Figure 3: A) Chessboard ROIs in 600 x 600 x 600 um volume B) ASAP labelling in the in M1 region, red squares mark the recorded areas with low resolution. C) Voltage traces from 13 neurons in head-fixed conditions recorded with 400 Hz speed.
- Dendritic voltage imaging
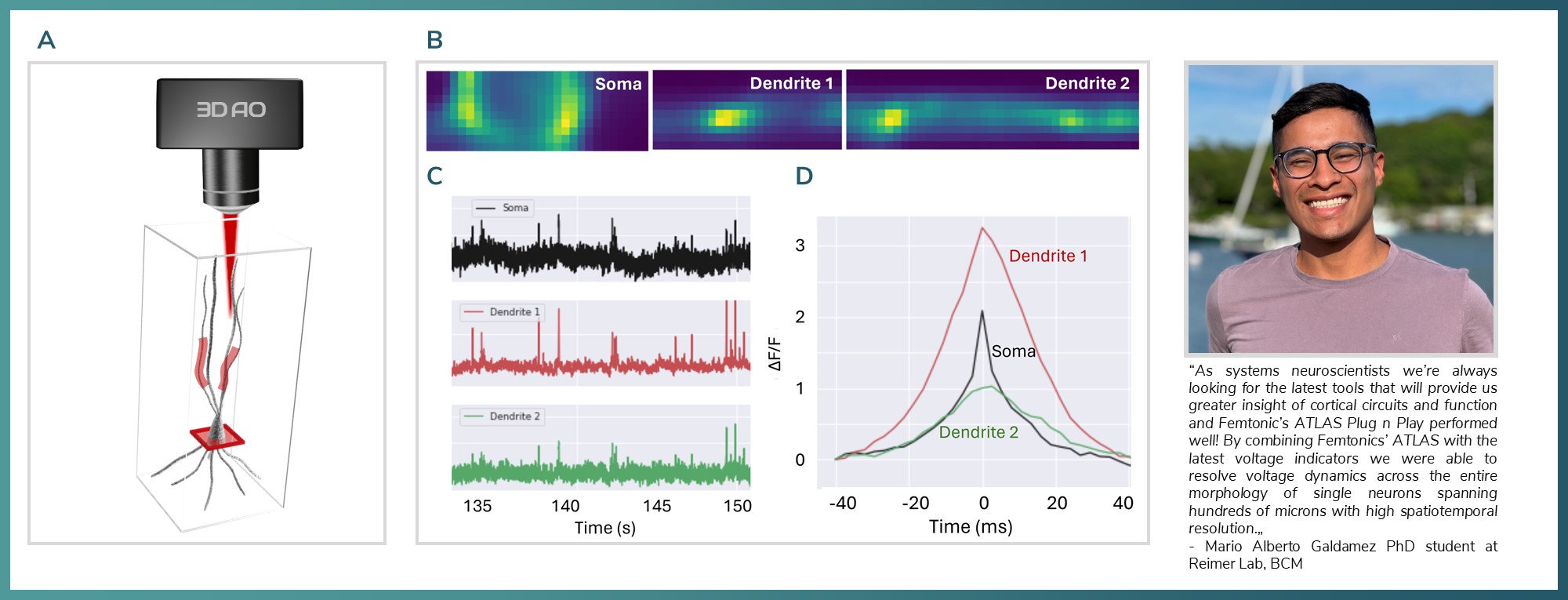
Figure 4: A) 3D model showing a chessboard and ribbon scans from the same neuron in visual cortex B) Recorded ROIs from different planes. C) Voltage traces the recorded areas D) Soma and the two different dendritic segment aligned.
- Simultaneous Ca and Voltage imaging measurements
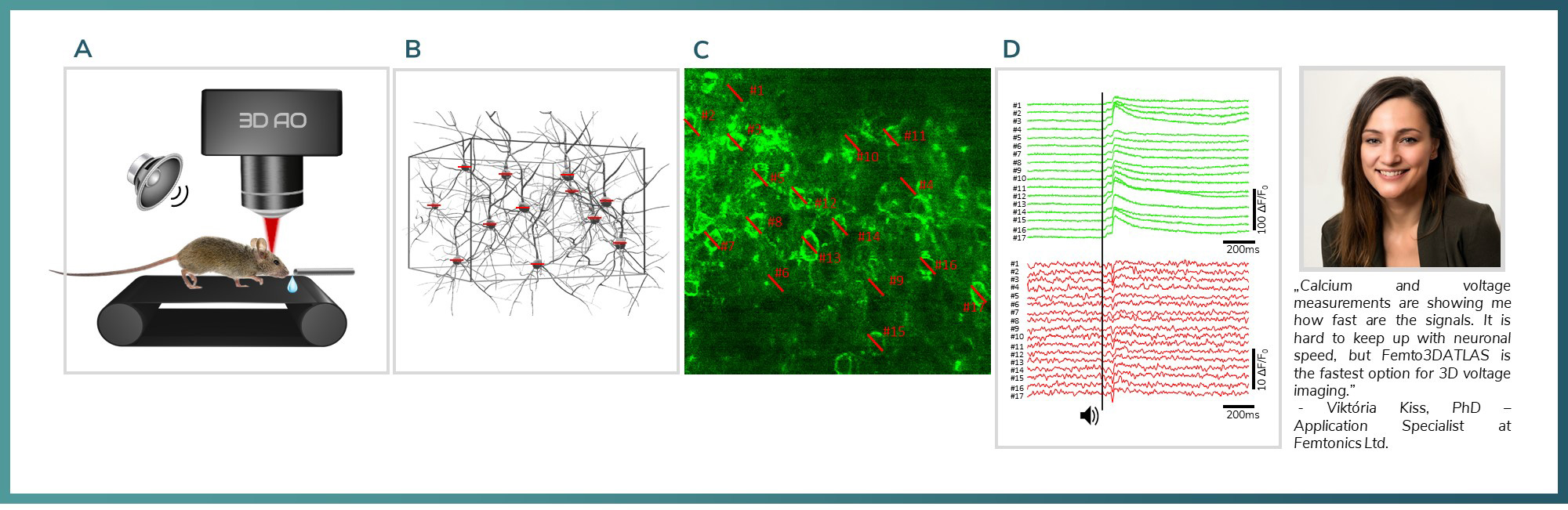
Figure 5: A) Experimental setup: mice were active on a treadmill while undergoing two-photon voltage imaging with Femto3DATLAS. Auditory stimulus served as activation signal in the V1 cortex. B) 3D model of the network which was recorded with line scans. C) JEDI2P labelled neurons in the field of view. Red lines mark the location of the line scans with 2.2kHz D) Averaged signal responses for the auditory stimulus from 17 neurons. Green: jRGECO calcium signals, Red: JEDI2P voltage signals excited on 1040nm. The black line shows the timing of the auditory stimulus.
- Optical electrophysiology in drosophila
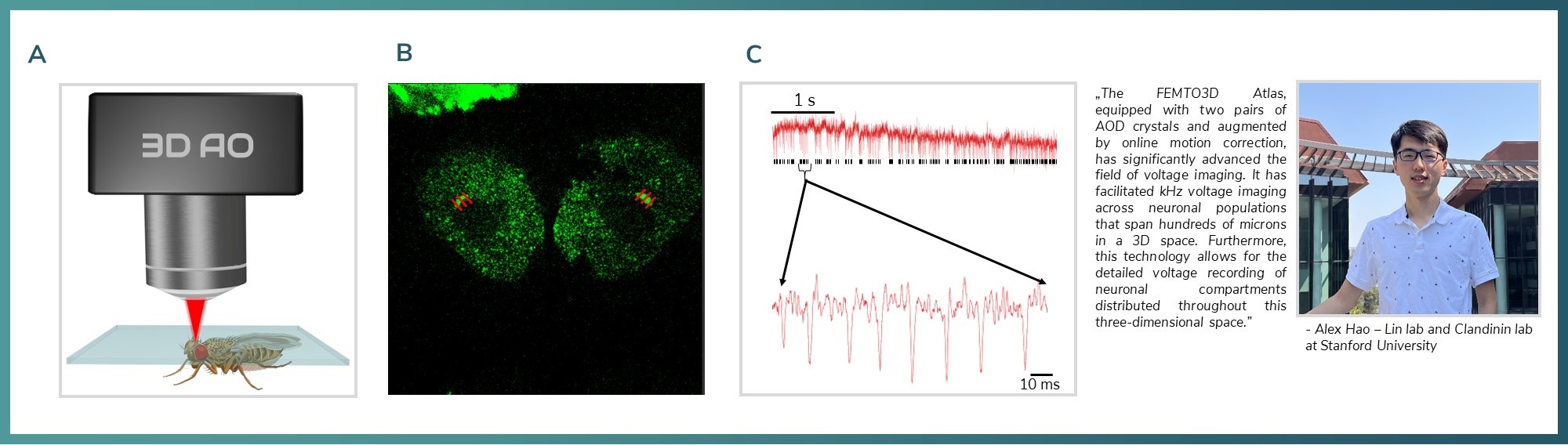
Figure 6: A) Schematics of Drosophila 2p imaging. B) ASAP labelled Drosophila neurons. Red lines over the somata are representing the line scan locations C) ASAP signal from the labelled neuron recorded with 6675.57 Hz.
2. In vitro voltage imaging with FEMTO3D Atlas
We offer optical electrophysiology solutions for problems that are impossible or close to impossible to solve with classical electrophysiology (i.e. multiple site dendritic patch, repeated bouton patch etc.) working today in the patch clamping world of electrophysiology and photoactivation, as multiple measurements sites can be recorded with up to 100 kHz by applying the DRIFT scanning in 3D. (Szalay et al. 2016)
Dr. Christopher D. Makinson – Assistant Professor of Neurological Sciences, Columbia University
- Optical validation of voltage sensor
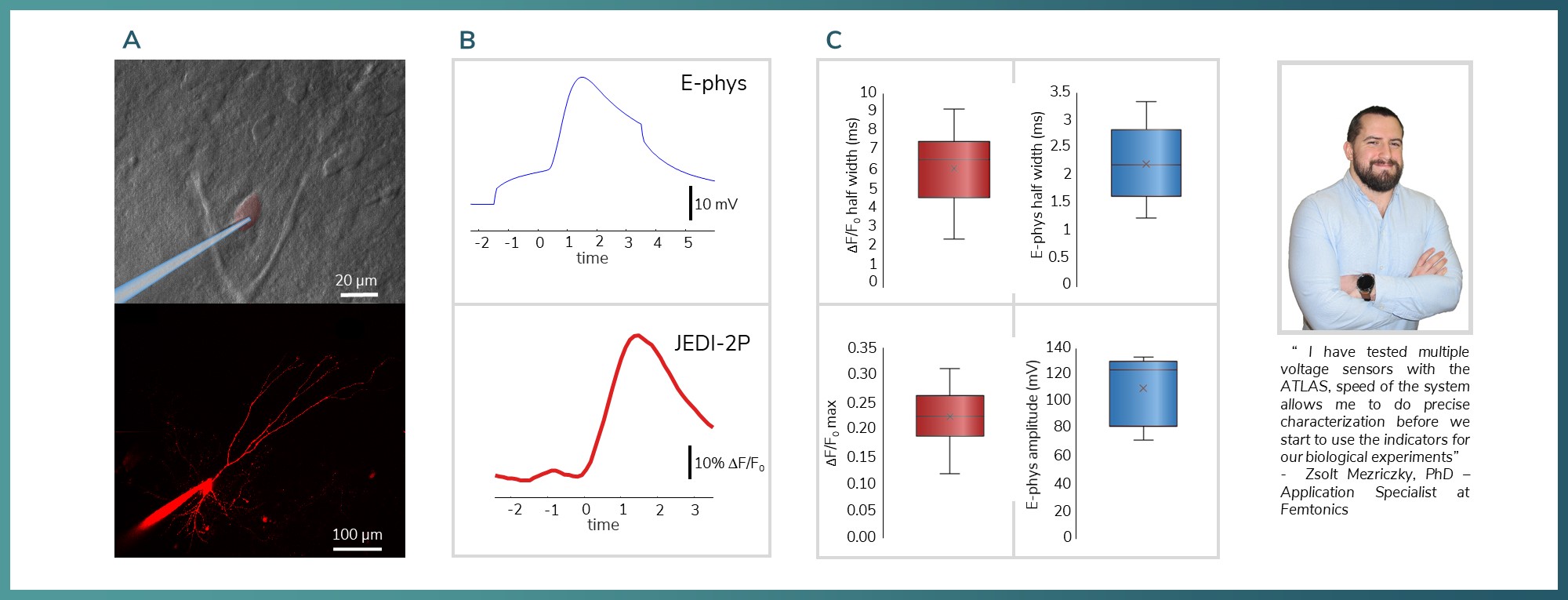
Figure 7: Validation of concept comparison of acousto-voltage imaging and electrophysiology measurements. A) Whole cell patch–clamp camera image and the maximum intensity projection of the patched neuron under two–photon microscopy B) Rise time comparison of the JEDI2P and the electrophysiological signal C) Half-width on the top and signal amplitude measurement comparisons on the bottom.
- Optical somatic electrophysiology of cell cultures, organoids, brain slices
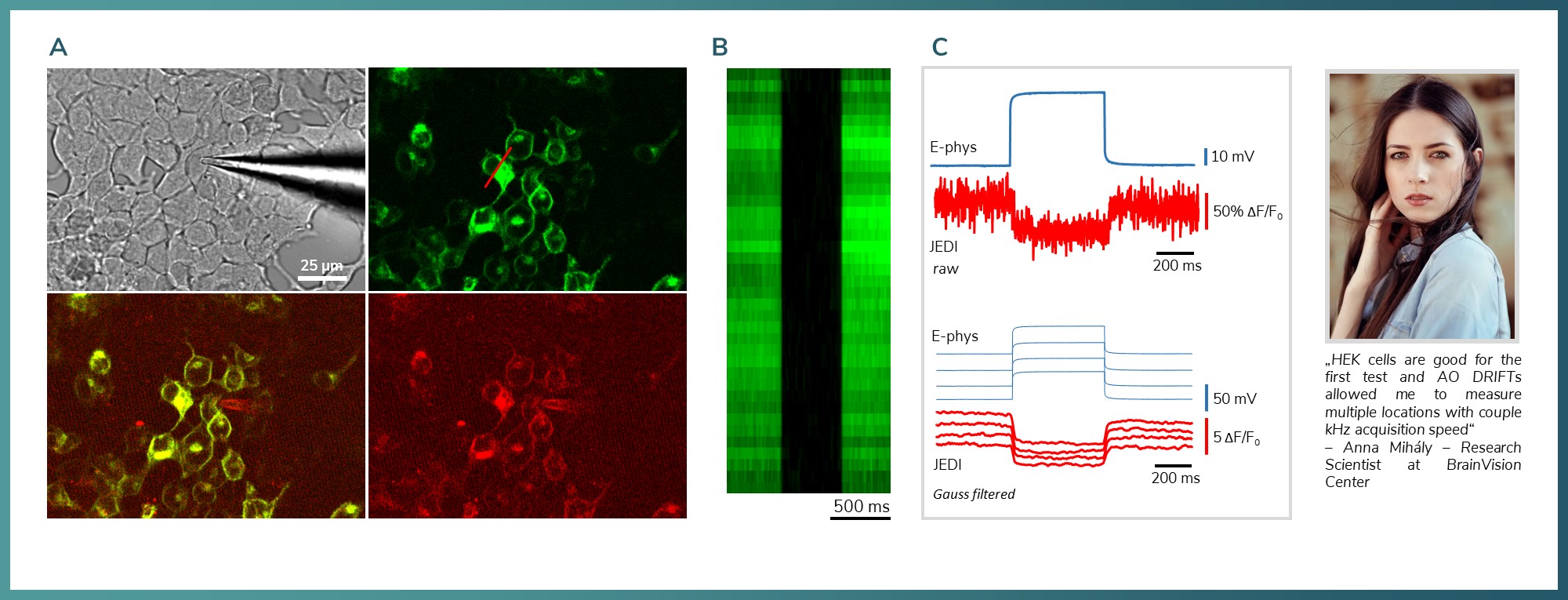
Figure 8: Combined two-photon and electrophysiology of HEK 293 cells. A) Camera image and fluorescent labelling of the field-of-interest. JEDI2P in the green channel. The measurements were done along the line marked with red. B) Fluorescent intensity change during ramp test on a HEK cell. The intensity decreased under the detection threshold C) Top is raw data, bottom is filtered. Somatically evoked ramp test. Blue: electrophysiology, red is voltage signals measured with 8.32 kHz.
- 3D Optical electrophysiology from dendrites and spines
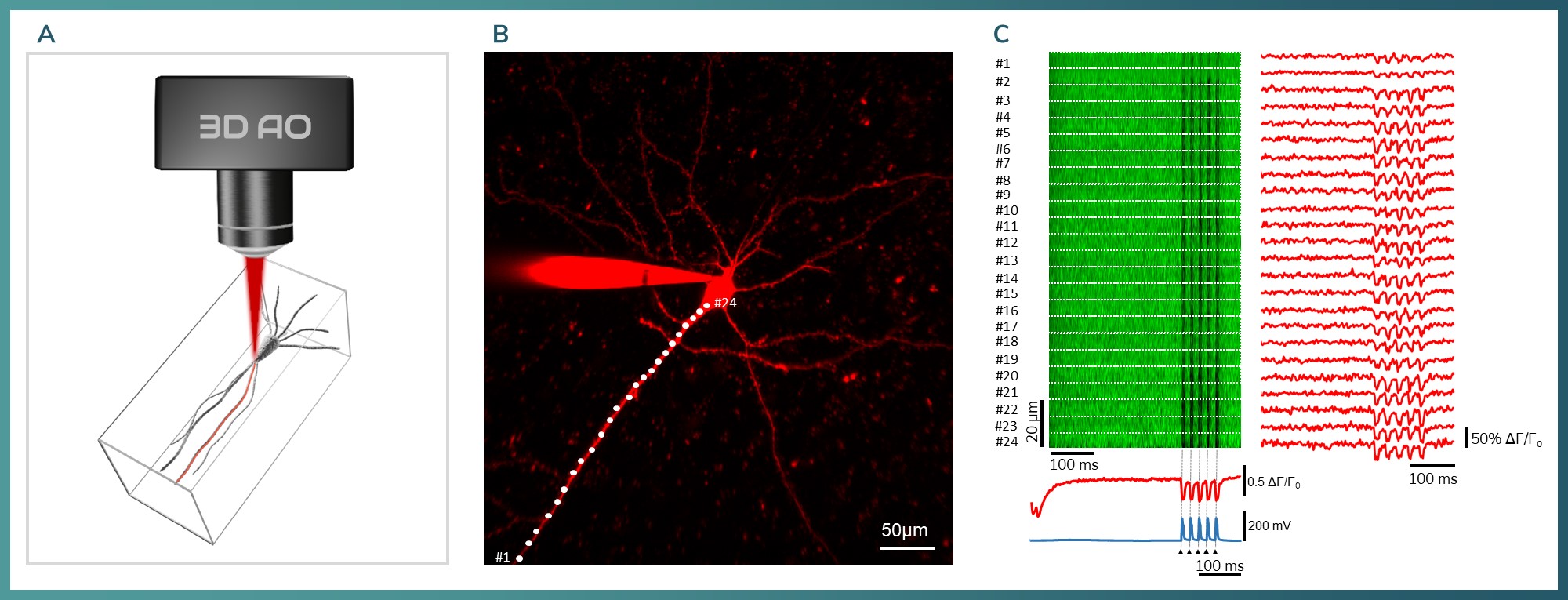
Figure 9: A) 3D model of a single pyramidal neuron measured in vitro with 3D line scan. The red mark is showing the recorded region with a 3D line scan. B) Maximum intensity Z projection of a cortical pyramidal cell. White dots are indicating the measurement locations. C) Somatically evoked 5 backpropagating action potentials. Left: Fluorescent intensity map of JEDI2P from pyramidal cell’s apical dendrite. Right: Temporal integration of the intensity map at the given ROI. There were 24 ROIs from the measured dendritic area with 2.24 kHz. Bottom: Arrows indicating the somatically evoked events, blue: electrophysiology, red: integrated curves for the whole dendrite based on the JEDI2P signal.
- Optical electrophysiology combined with uncaging
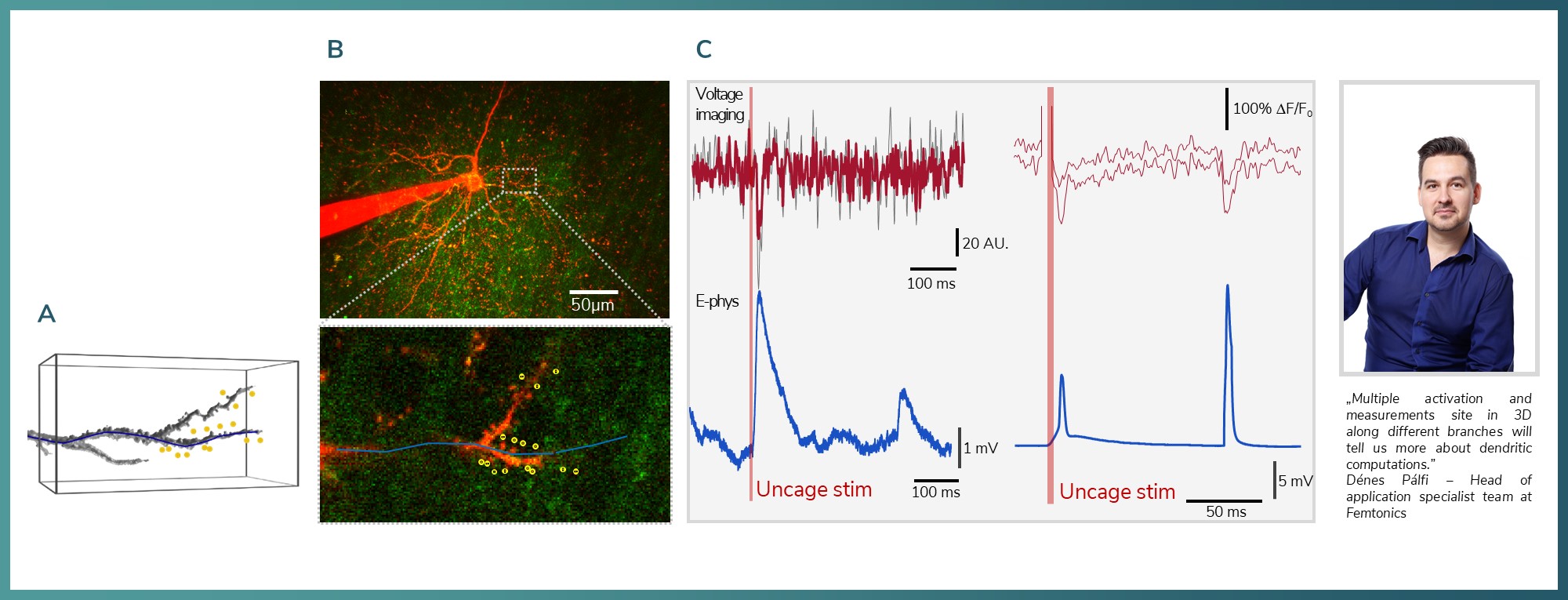
Figure 10: A) 3D reconstruction of a dendrite with the uncaging locations marked with yellow. The imaging as 3D line scan marked with blue B) Maximum Z-projection of a pyramidal neuron (ALEXA-594) and in the inset the scanning line (blue), uncaging locations (yellow, 15 locations, 0.3 ms ROI time). C) Examples of uncaging (DNI-Glu) evoked EPSP (top, JEDI-2P signal on dendrite, bottom somatically recorded electrophysiological signal).
Reference labs are located at: Stanford University, Baylor College of Medicine, Columbia University, Basel University, NIH, and BrainVisionCenter.
We are eager to demonstrate our capabilities online in vitro or in vivo. Book an appointment to see how easy it is to achieve such results.
More developments are in the works that focus on voltage imaging such as disc scanning, 3-level image stabilization, immediate multi-channel analysis, etc. Our partners have an extremely high grant success rate with voltage imaging. We are ready to help you and work with you on your grant applications: CONNECT to you sales representative for grant application assistance!
Network Imaging
The FEMTO3D Atlas microscope with its unique technical solutions enables the precise spatial and real-time complexity of neuronal coding to be resolved by scanning large numbers of cells distributed in a 3D volume. Imaging of thousands of cells simultaneously with maximal scanning speed (100 kHz), high signal-to-noise ratio, and real-time motion correction in 3D is now possible. The system allows quasi-simultaneous 3D photostimulation and all features are integrated into the Femtonics two-photon imaging platform.

3D Imaging Modes:
- 3D random-access point scanning – ultra-fast measurements in selected regions
- 3D chessboard scanning – high-resolution imaging during behavior
- 3D multi-cube scanning – motion-compensated volume acquisition
- High-speed arbitrary frame scanning – plane rotation in 3D

Featured highlights:
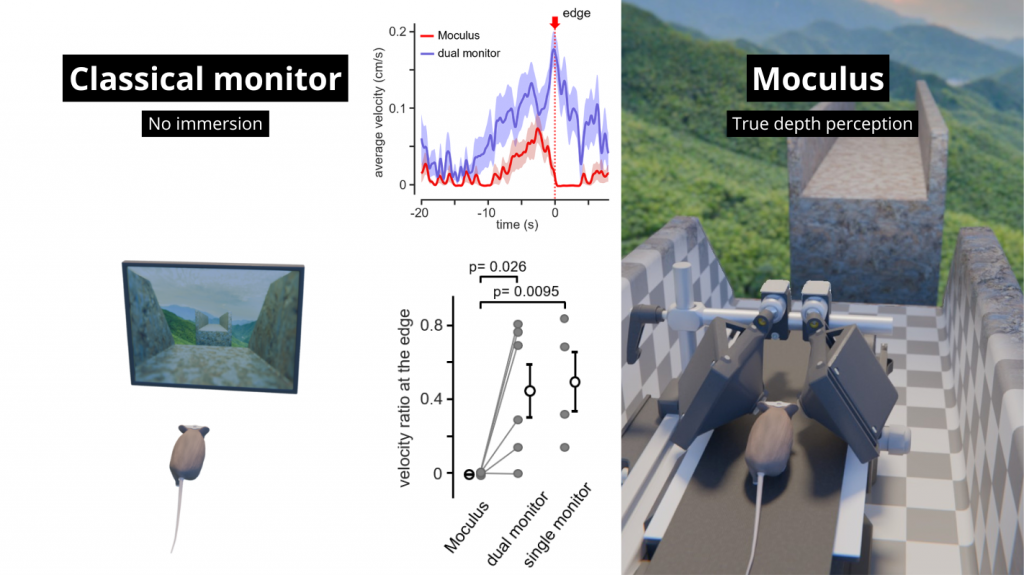
- Full immersion
- Enables rapid visual learning
- Ready-to-use setup right out of the box
- Highly customizable virtual environment
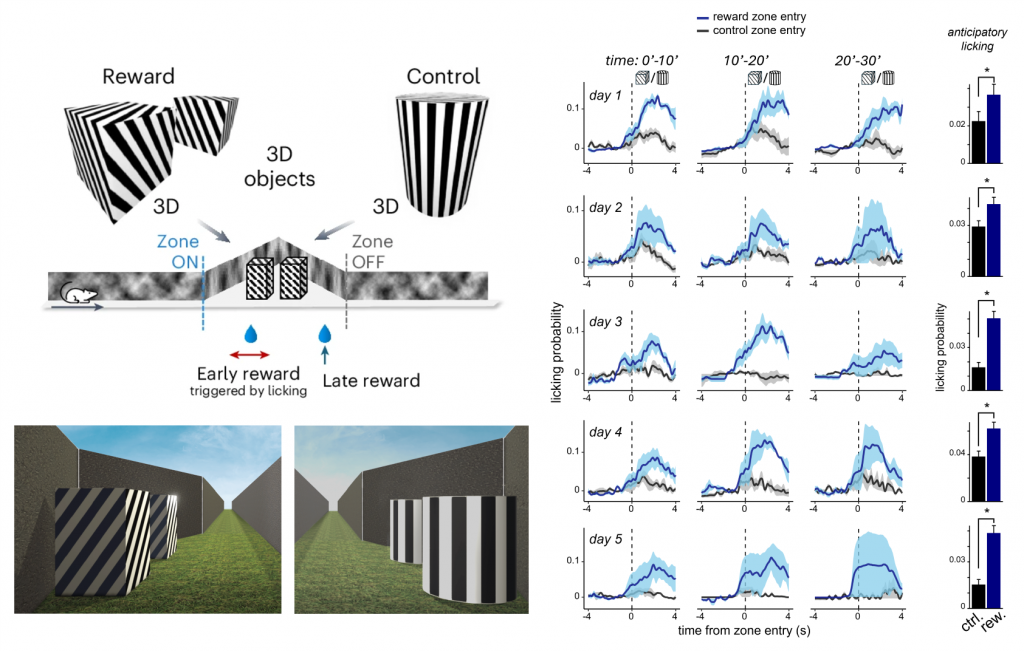
- Up to 300x faster learning speed compared to 2D monitor systems
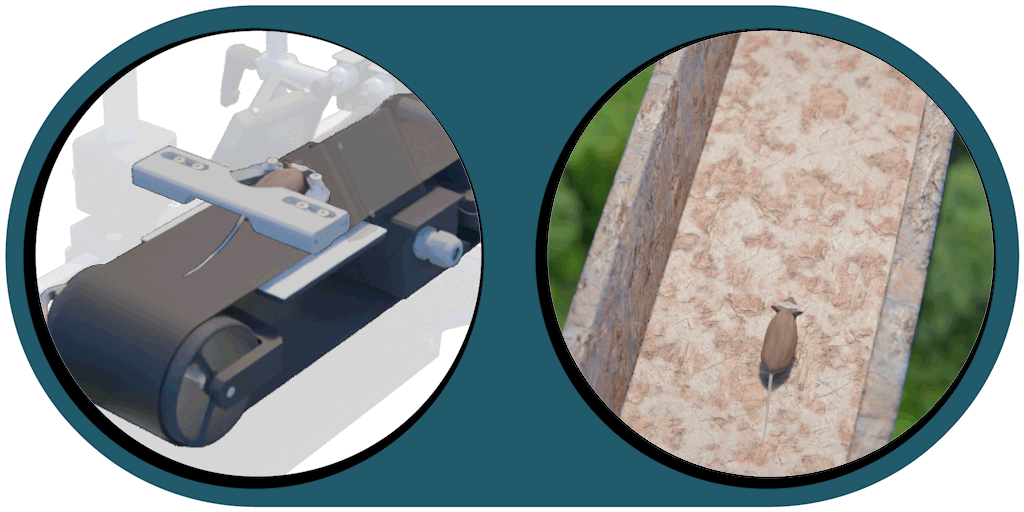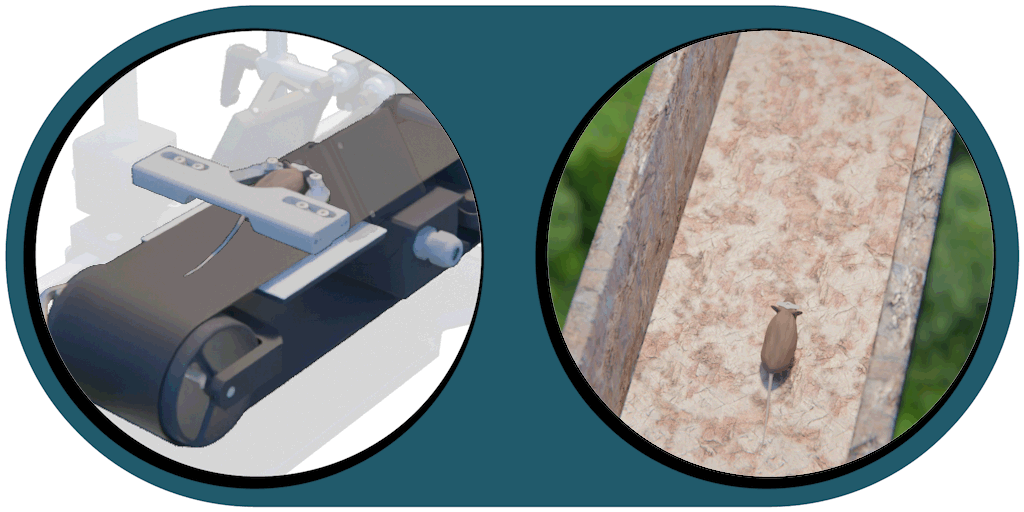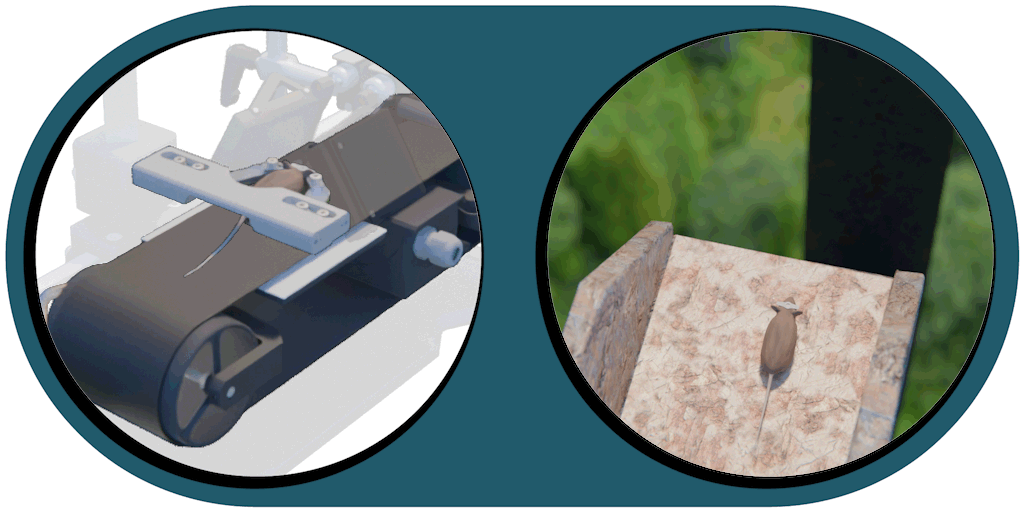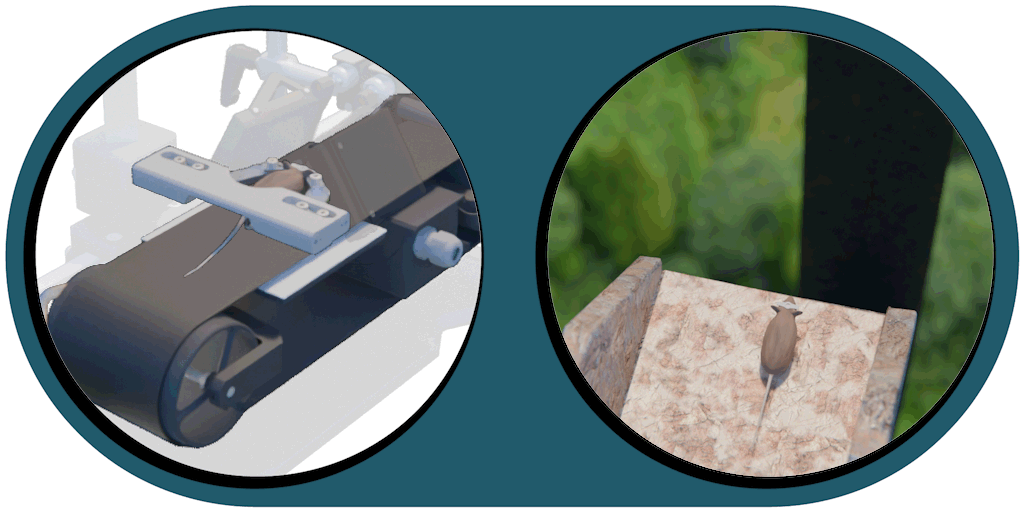
Great projection accuracy in the binocular zone
enabling true stereo vision
Enables investigating innate behaviors without
training (e.g. abyss test, looming disk stimulus)
Key specifications:
- Binocular vision
- Behavior feedback contributing to sense of presence
- Natural reaction to the virtual environment without preconditioning
- Modular & Scalable – Seamlessly integrate with two-photon imaging and electrophysiology setups
- Creating realistic virtual environments and behavioral protocols that emulate the animal’s natural world, using a state-of-the-art engine
Click on a category to explore:

Flexible Experiment Design – Create fine-tuned virtual environments
for complex studies
Fast visual discrimination learning by covering
large area of the field of view
Innate depth aversion is immediately achieved with Moculus without any prior training
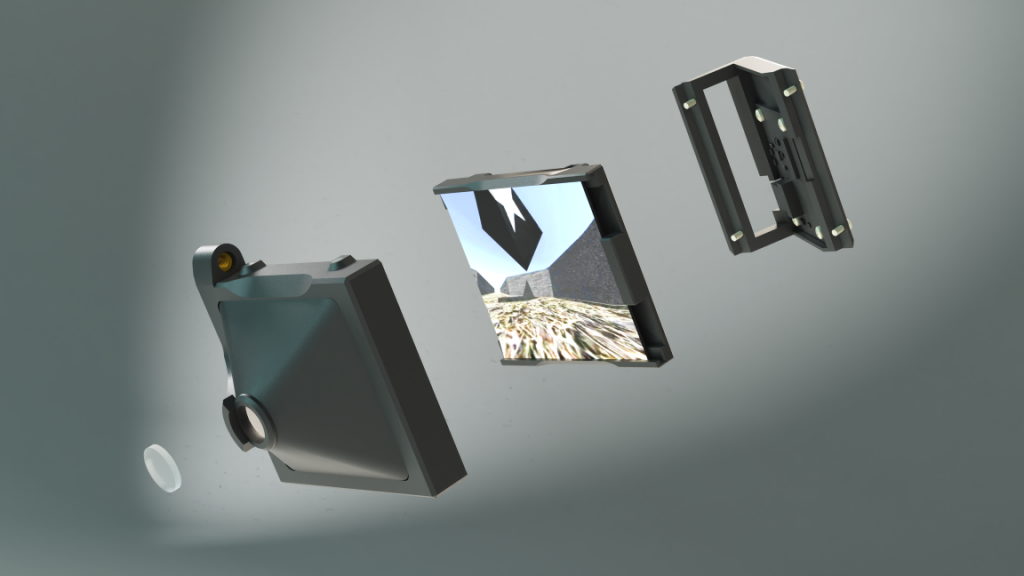
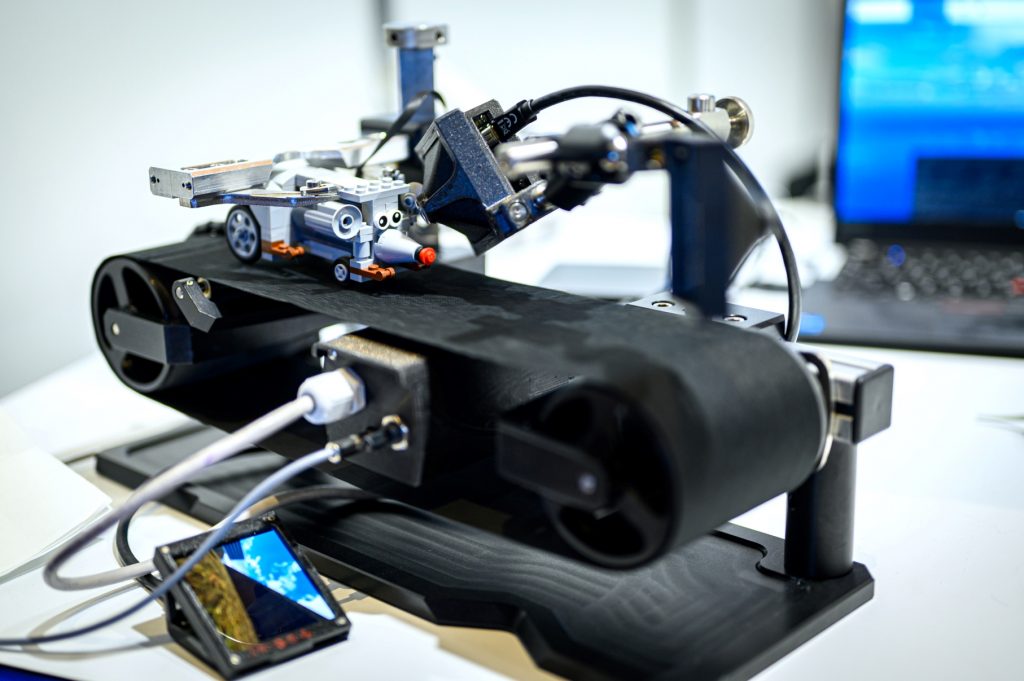
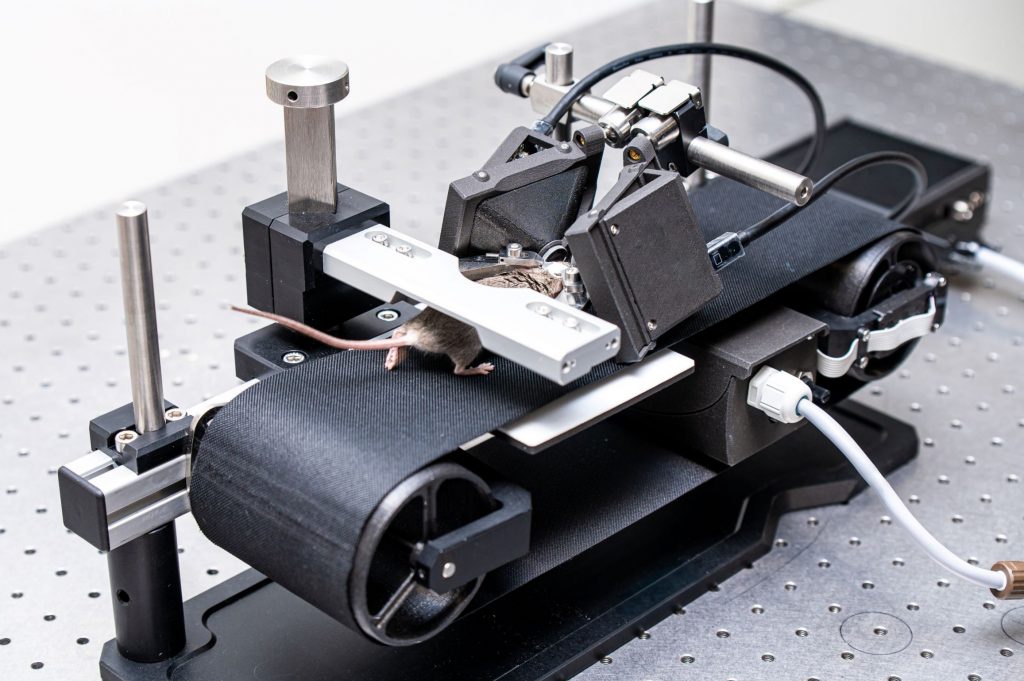
“Sense of presence” – when animals perceive VR as reality
Facilitating high-resolution insights
Natural and unbiased behavior under precise head-fixed conditions
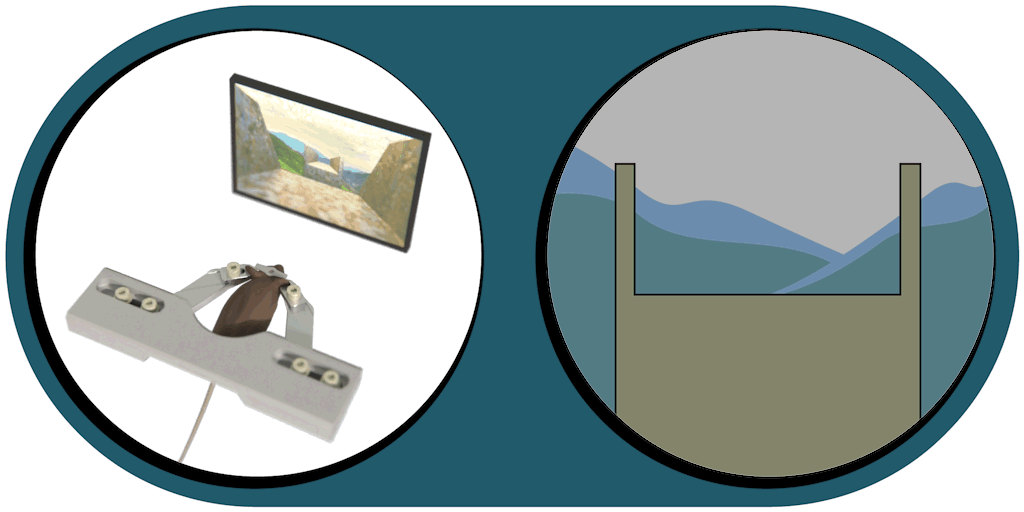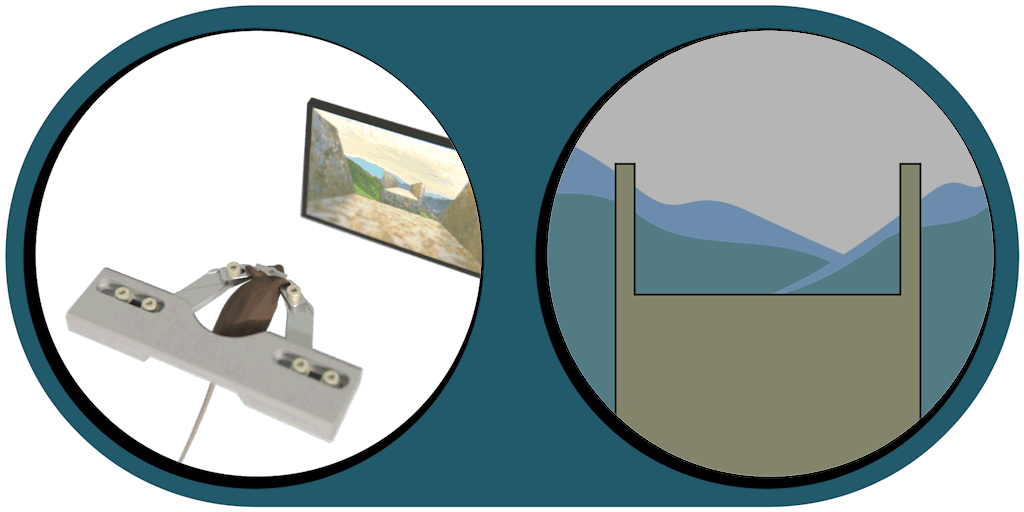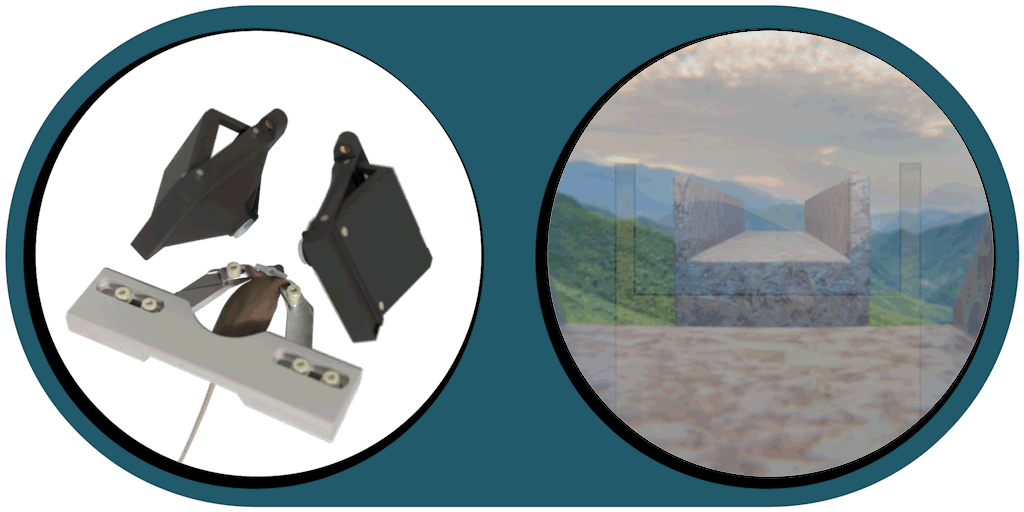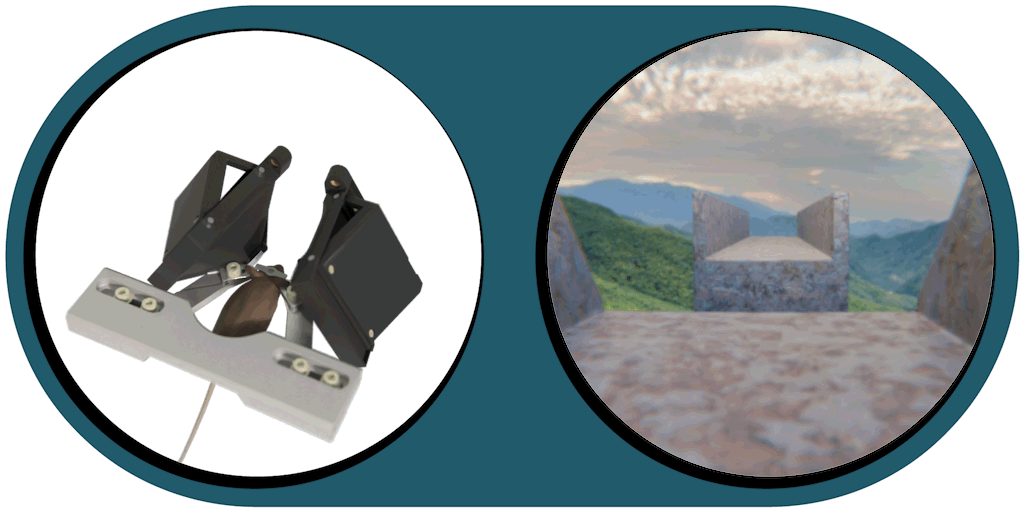
Virtual Reality Kit
Our patented comprehensive head-aligned VR display for mice provides binocular depth perception and stereoscopic 3D vision. By positioning the displays to the eyes of a head-fixed mouse running on a treadmill, virtual scenes can be displayed to the mouse in closed-loop experiments, where the treadmill motion controls the visual scene. This makes it possible to reproduce real, freely moving behavior tasks, whilst the high-resolution, large FOV or even 3D random-access microscope records the brain states on the cellular or subcellular level. The photorealistic rendering engine creates perspective corrected 3D virtual world and stereoscopic binocular vision in a large FOV, allowing full immersion in any imaginable environment.
The biggest added value of Virtual Reality is the sense of presence, when the experimental animal perceives the widely controllable and reproducible virtual environment around it as reality itself.
- Behavior feedback contributing to presence
- Binocular vision with depth perception, full immersion
- Natural reaction to the virtual environment without preconditioning
- Modular and scalable design for two-photon imaging and electrophysiology
- Covers most of the field-of-view without static visual cues in the FOV which might disturb presence
- Photorealistic rendering with a state-of-the-art engine to flexibly design your own virtual environments and behavioral protocols
Technology Overview
To better understand brain functions during natural behavior, experiments that mimic the real world as closely as possible are essential. High-resolution imaging requires head fixation to the microscope, enabling subcellular imaging of thousands of cells with advanced 3D scanning and photostimulation, unlike free-running animal studies with limited FOV and resolution. Neuronal activity of cells can be followed for months in accordance with behavioral experiments. Virtual-reality (VR), in combination with head-fixed recordings, provides control and stability for recordings during behavioral experiments enabling the creation of complex interactive virtual environments, allowing every aspect of the visual input of the experimental subject to be controlled. The Mouse Virtual Reality system provides the animal with a close to real life sensation, thereby achieving faster learning and evoking non-trained, lifelike innate behavioral reactions. While habituation is still required to both the treadmill and the Moculus displays, innate behaviors (e.g. escape behavior from a looming stimuli) can be investigated without any previous training. Also, while fast learning was achieved with non-naïve, pretrained mice, the ability to investigate learning processes within the same day opens new possibilities in exploring the neuronal mechanisms of memory formation and visual processing related network changes.
While habituation is still required to both the treadmill and the Moculus displays, innate behaviors (e.g. escape behavior from a looming stimuli) can be investigated without any previous training. Also, while fast learning was achieved with non-naïve, pretrained mice, the ability to investigate learning processes within the same day opens new possibilities in exploring the neuronal mechanisms of memory formation and visual processing related network changes.
Developed by BrainVisionCenter Nonprofit Ltd. Research Institute and Competence Centre
Manufactured by Femtonics Ltd.