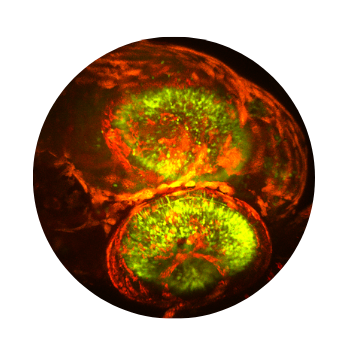
Voltage Imaging
Reference1: Gonzalez et al. (2025)
Reference2: Yukun A. et al. (2024)
Download your whitepaper on: VOLTAGE Imaging, 3D Real-Time Motion Correction
Network Imaging

All sensations and behaviors arise from dynamic neural network activity spanning multiple cortical layers. Two-photon microscopy now enables access to deep brain regions (up to ~850 µm) with high spatiotemporal resolution. For voltage imaging, the Femto3D Atlas provides fast, flexible scanning strategies—such as line scanning for capturing rapid events and chessboard scanning for parallel monitoring of distributed neurons—allowing precise measurement of network dynamics in 3D. Learn more
Reference1: Fiona E. Müllner et al. (2024)
Reference2: Geiller et al. (2022)
Download your whitepaper on: 3D Real-Time Motion Correction
Dendritic Imaging

Dendrites and spines are thin, elongated structures that are challenging to study. Two-photon microscopy enables deep-brain imaging with minimal phototoxicity, while regional scanning of axons, dendrites, and spines captures even subthreshold signals with high SNR. The Femto3D Atlas offers multiple configurations—including ribbon and trajectory scanning—to visualize dendritic arborization and perform precise functional measurements in vivo and in vitro. With 3D Real-Time Motion Correction, motion artifacts in dendritic imaging are effectively eliminated, even in behaving animals. Learn more
Reference1: Fiona E. Müllner et al. (2024)
Reference2: Geiller at al. (2022)
Download your whitepaper on: 3D Real-Time Motion Correction
Behavioral Studies

Understanding CNS function requires studying neural activity during behavior, where variables such as stimuli, tasks, rewards, and aversive outcomes directly shape responses. The Femto3D Atlas is designed for such experiments, enabling two-photon imaging in awake, behaving animals at kilohertz speeds with real-time motion correction for high-SNR signals. Its compatibility with advanced behavioral setups—including virtual reality systems like Moculus—allows precisely controlled, naturalistic environments. This makes it possible to monitor locomotion, decision-making, learning, and memory while simultaneously imaging hundreds of neurons, as demonstrated in mice during random foraging, zebrafish with optogenetic stimulation, and Drosophila in memory paradigms. Learn more
Reference1: Linda Judák et al. (2025)
Reference2: Zoltán Szadai et al. (2022)
Reference3: Yukun A. Hao et al. (2024)
Download your whitepaper on: Behavioral Experiments
3P Imaging

Three-photon (3P) microscopy enables noninvasive structural and functional imaging deep within tissue, offering higher contrast and spatial resolution than two-photon excitation. Its optical design provides superior axial resolution, while the longer-wavelength spectral window allows excitation of diverse fluorophores, including modern protein-based calcium indicators such as GCaMP. With laser repetition rates optimized for detecting Ca²⁺ transients, 3P microscopy is ideally suited for capturing neural activity at depth. Learn more
FLIM

Fluorescence-lifetime imaging microscopy – FLIM
Fluorescence-lifetime imaging microscopy (FLIM) provides both structural intensity maps and functional lifetime data, while minimizing photon scattering in thick samples. It yields quantitative measurements of molecular environments, interactions (via FRET), and cellular metabolism, independent of emission strength or fluorophore distribution. Two-photon FLIM (2P-FLIM) further enhances neuroscientific applications with deep penetration, reduced photobleaching, and intrinsic optical sectioning. Recently, successful efforts have combined FLIM with voltage imaging, opening new possibilities to simultaneously track membrane potential dynamics and cellular microenvironment with high precision. Learn more
Reference1: Yukun A. Hao et al. (2024)
Reference2:
Excerpt from: Tyurikova, O., Kopach, O., Zheng, K., Rathore, D., Codadu, N., Wu, S. Y., … & Rusakov, D. A. (2025). Astrocyte Kir4. 1 expression level territorially controls excitatory transmission in the brain. Cell Reports, 44(2).
Blood Flow
Following blood flow deep in the living tissue
Abnormal hemodynamics are implicated in disorders such as stroke and Alzheimer’s disease, yet understanding these links requires precise tools to measure blood flow in vivo in 3D. Acousto-optic multiphoton microscopy enables high-resolution tracking of blood flow at the cellular level, even deep within living tissue. With its unique scanning modes, the FEMTO3D Atlas can record continuously along complex 3D trajectories, while extensions allow simultaneous monitoring of multiple parameters, including blood flow and cellular activity. These capabilities open powerful applications across neuroscience, immunology, dermatology, oncology, and beyond. Learn more
Reference1: Changsi Cai et al. (2023)
Download your whitepaper on: Blood Flow Tracking and Analysis
3D Photostimulation

Optogenetics uses light-sensitive ion channels or pumps such as channelrhodopsin or halorhodopsin to selectively activate or silence neurons. The FEMTO3D Atlas Dichro enables temporally and spatially precise 3D optogenetic stimulation alongside calcium (or voltage) imaging, using dual wavelength lasers and acousto-optic scanning for flexible targeting of single cells, dendrites or other cellular constituents. Photostimulation can be delivered at submillisecond resolution across many sites, with rapid laser switching (tens of microseconds) ensuring seamless alternation between imaging and stimulation. A built-in gating system protects detectors during stimulation, while the single scan-head design keeps the setup efficient and easy to align. Learn more
Reference1: Kaszas A. et al. (2021)
Download your whitepaper on: Photostimulation and Uncaging with AO
Uncaging

Uncaging refers to the light-triggered release of biochemically masked molecules, mimicking the natural release of neurotransmitters such as glutamate. In neuroscience, two-photon photostimulation with ultrafast pulsed IR lasers enables highly localized uncaging in tiny volumes, providing precise spatial and temporal control. Combined with two-photon imaging, this approach allows researchers to track dendritic and spine responses and even investigate receptor distribution on neurons with subcellular resolution. Learn more
Reference1: Balázs Chiovini et al. (2021)
Download your whitepaper on: Photostimulation and Uncaging with AO
Electrophysiology

Two-photon Ca²⁺ imaging and electrophysiological recordings are complementary tools for studying neural cell and network activity. Together, they allow fluctuations in membrane potential to be directly linked to Ca²⁺ dynamics. Two-photon Ca²⁺ imaging offers submicron spatial resolution, capturing events in dendrites and spines, but is limited in temporal resolution (>100 ms). Electrophysiology, by contrast, achieves millisecond precision (<1 ms) across larger areas, though with lower spatial resolution (~150 µm). While electrophysiology remains indispensable, recent advances in optical electrophysiology—combining the speed of voltage indicators with the spatial reach of optical methods—are bridging this gap, enabling high-resolution, high-speed functional imaging of neural activity. Learn more
Reference1: Balázs Chiovini et al. (2021)
Download your whitepaper on: VOLTAGE Imaging, Discover our Applications flyer
Deep Functional Imaging

A key advantage of two-photon microscopy is its ability to probe not only the surface but also deep layers of brain tissue, far beyond the reach of confocal microscopy, which is limited to just a few dozen micrometers. The achievable depth depends on multiple factors—laser wavelength and type, labeling quality, tissue properties, and preparation. Advanced imaging strategies further extend these limits, with techniques such as laser beam manipulation and the use of GRIN lenses enabling functional imaging beyond 1 mm below the surface. With these approaches, our Deep Functional Imaging platform pushes the boundaries of in vivo visualization, making it possible to study neural circuits at unprecedented depths. Learn more
Reference1: Linda Judák et al. (2022)
Download your whitepaper on: Deep Functional Imaging
Mouse Imaging

Mice are a central model for neuroscience, offering direct links between cellular mechanisms and complex behavior. The FEMTO3D Atlas allows large-scale, high-speed imaging of cortical and subcortical networks in awake animals. Combined with Moculus VR, naturalistic yet fully controlled behavioral environments can be created, enabling researchers to link neuronal dynamics with locomotion, sensory processing, decision-making, and learning.
Reference1: Soledad Gonzalo Cogno at al. (2024)
Download your whitepaper on: Behavioral Experiments
Drosophila Imaging

Drosophila provide an ideal system for dissecting neural circuits with genetic precision. The FEMTO3D Atlas enables rapid voltage and calcium imaging with submicron spatial resolution, while advanced scanning strategies such as line and ribbon scanning allow detailed interrogation of dendrites, spines, and small neuronal populations. This makes it possible to uncover fundamental principles of synaptic integration, sensory coding, and plasticity at high temporal resolution.
Reference1: Laurie Cazalé-Debat at al. (2024)
Download your whitepaper on: Behavioral Experiments
Zebrafish Imaging

Zebrafish are highly suitable for developmental and systems neuroscience due to their transparency and genetic tractability. The FEMTO3D Atlas supports deep, in vivo imaging across developmental stages, enabling time-lapse and volumetric recordings of neural activity. By capturing both anatomical structure and functional dynamics in intact animals, researchers can study how circuits form, reorganize, and generate behavior over time.
Reference1: Marco dal Maschio at al. (2017)
Download your whitepaper on: Behavioral Experiments
Organoid Imaging

Organoids provide powerful in vitro models for studying human development, disease, and neural network formation in a controlled 3D environment. The FEMTO3D Atlas enables deep, high-resolution imaging of intact organoids, capturing cellular organization, calcium dynamics, and network activity across large volumes. Its acousto-optic scanning and precise 3D targeting allow long-term, minimally invasive monitoring of functional maturation and drug responses. By combining optical sectioning, rapid volumetric imaging, and compatibility with electrophysiological or optogenetic approaches, the system bridges the gap between cellular-level studies and complex tissue modeling.
Reference1: Cameron S. Cowan at al. (2020)
GRIN-lens Imaging

GRIN (Gradient Refractive Index) lens imaging enables optical access to deep brain regions by inserting a gradient lens that focuses light below the cortical surface. FEMTO3D Atlas microscopes integrate acousto-optic scanning with GRIN lenses to achieve fast (up to 100 kHz) 3D in vivo imaging through the implanted lens, even during behavior, without requiring further lens movement. This method allows stable, high-resolution observation of neural activity within volumes of ~200 × 200 × 230 µm, reaching depths of up to 8000 µm below the surface. In addition, GRIN lens imaging minimizes photodamage, preserving tissue integrity while providing clear visualization of deep brain structures.
Reference1: Lior Matityahu at al. (2023)
Time-lapse imaging
Time-lapse imaging enables continuous monitoring of biological processes, capturing structural and functional changes over time. In in vivo experiments, even subtle motion can distort long recordings; the FEMTO3D Atlas addresses this with its integrated 3D Real-Time Motion Correction (3D-RTMC), which actively compensates for movement in real time. This ensures stable, precisely aligned data across extended imaging sessions. Combined with automated acquisition protocols and low-phototoxicity optics, the system provides reliable, high-quality time-lapse recordings of neural activity, plasticity, and long-term functional changes.
Marmoset imaging

Marmosets are a valuable primate model for exploring complex brain functions such as perception, cognition, and social interaction. Femtonics two-photon microscopes enable high-resolution imaging of cortical and subcortical activity in awake or anesthetized marmosets. Their flexible design and acousto-optic scanning allow rapid 3D targeting across large neural populations while maintaining stability during behavioral or head-fixed experiments. When using techniques such as optogenetics, calcium imaging, or voltage imaging, these microscopes provide precise, deep-tissue measurements that bridge cellular dynamics and systems-level function in the primate brain.
Reference1: Sheng-Hui Zhang et al. (2024)
Download your whitepaper on: Behavioral Experiments
Excerpt from: Ju, N. S., Guan, S. C., Tang, S. M., & Yu, C. (2022). Macaque V1 responses to 2nd-order contrast-modulated stimuli and the possible subcortical and cortical contributions. Progress in Neurobiology, 217, 102315.
Closed-loop imaging

Closed-loop imaging enables real-time adjustment of imaging parameters based on external inputs or ongoing experimental events. In this dynamic approach, imaging begins, a change—such as a behavioral response, stimulus, or neural signal—is detected, and the system reacts immediately. The FEMTO3D Atlas can modify imaging settings, shift photostimulation patterns, or redefine regions of interest on the fly. This integration of feedback-driven control allows researchers to directly link behavior, stimulation, and neural activity, opening the way for fully interactive experimental designs.
Download your whitepaper on: Our New Developments flyer
Unique species
The versatility of the FEMTO3D Atlas extends beyond standard model organisms, enabling imaging across a wide range of species and preparations. Its spacious shading box and adaptable optical design allow flexible experimental configurations, accommodating diverse sample geometries and physiological setups. This flexibility makes it possible to perform precise, high-speed two-photon imaging even in uncommon models—such as monitoring the beating salamander heart—while maintaining optimal stability and signal quality. The system’s compatibility with various objectives, detectors, and behavioral setups ensures seamless integration into specialized research environments.