
The Innovation Award winner FEMTO3D Atlas combines high-tech science and engineering in 3D measurements. It implements and goes beyond the traditional galvo and resonant scanner-based imaging functions and combines them with a unique fast 3D imaging feature, providing an All-in-one solution in two-photon microscopy. The FEMTO3D Atlas enables its users to scan cells and neuronal, dendritic, or other biological processes in 3D, up to a million times faster than classical scanning methods, at a preserved two-photon resolution.
Watch our webinar about the FEMTO3D Atlas multiphoton microscope.
Neural networks consist of thousands of neurons dispersed in 3D space, often across many cortical layers of the brain tissue. 3D random-access scanning performed by AO technology combined with two-photon microscopy is the best choice to reveal the dynamics of the neural networks while processing information. It enables you to record neuronal activity from cell populations consisting of up to thousands of cells in vivo. The 3D anti-motion technology combined with new scanning methods united in FEMTO3D Atlas provides a flexible solution to gather motion-corrected data in large volumes at high framerates during behaviour. Learn more about Network imaging.
Watch the interview and find out how the FEMTO3D Atlas microscope contributed to the Losonczy Labs’ research published in Neuron.
Our application specialists are always ready to introduce you to the setup and answer your questions.
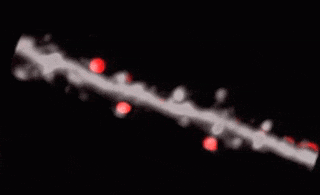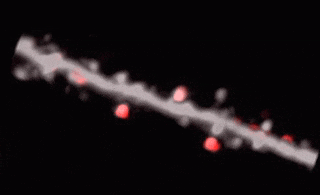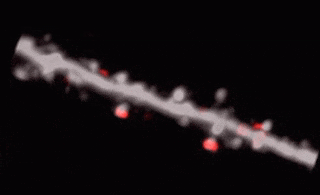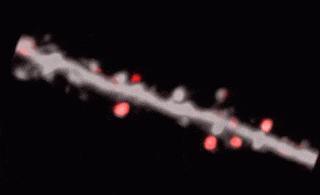
3D random-access point scanning extended by drifting the focal point along scanning elements with various shapes and sizes allows imaging without interruption at multiple dendritic branches. The sampling is continuous during the drift, so this scanning mode gives more detailed spatial resolution without changing the overall scanning time which reminds as high as during point scanning. As a result, the function of thin dendritic segments or even spines, single action potentials can be brought to light. See more Chiovini et. al, Neuron (2014).
 Our anti-motion technology enables cell activity to be captured while an animal is moving in virtual reality and performing tasks. To preserve signals while imaging, scanning points are extended to drifted lines which are precisely fitted to each other resulting in surface or volume elements enclosing the target object. These elements cover not only the pre-selected ROIs but also the neighboring areas giving an opportunity for motion correction by preserving all fluorescent information during motions and decreasing the artefacts by more than one order of magnitude in behaving animals.
Our anti-motion technology enables cell activity to be captured while an animal is moving in virtual reality and performing tasks. To preserve signals while imaging, scanning points are extended to drifted lines which are precisely fitted to each other resulting in surface or volume elements enclosing the target object. These elements cover not only the pre-selected ROIs but also the neighboring areas giving an opportunity for motion correction by preserving all fluorescent information during motions and decreasing the artefacts by more than one order of magnitude in behaving animals.
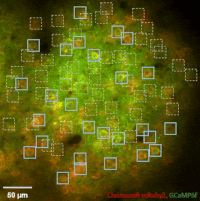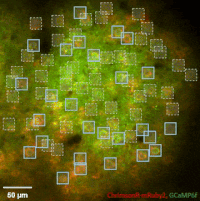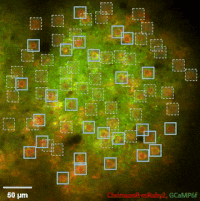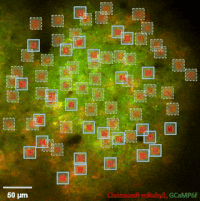 Photostimulation techniques allow activating cells in a selective and precise manner, which makes them advantageous for various biological applications. Enhancing two-photon microscopy with this capability makes this technology well suited for studies ranging from synaptic plasticity to experiments investigating learning and memory in vivo in the brain tissue. To the FEMTO3D Atlas Dichro extension, a second laser can be coupled enabling users to perform optogenetic stimulation or uncaging with calcium imaging, simultaneously, even in different cell populations.
Photostimulation techniques allow activating cells in a selective and precise manner, which makes them advantageous for various biological applications. Enhancing two-photon microscopy with this capability makes this technology well suited for studies ranging from synaptic plasticity to experiments investigating learning and memory in vivo in the brain tissue. To the FEMTO3D Atlas Dichro extension, a second laser can be coupled enabling users to perform optogenetic stimulation or uncaging with calcium imaging, simultaneously, even in different cell populations.
Using two-photon microscopy enhanced by the AO technology in combination with our easy-to-use data acquisition software package provides maximal flexibility in the fast selection of 3D ROIs with various shapes and sizes such as points, trajectories, squares, etc. The preselected ROIs can be precisely and rapidly targeted in the tissue without wasting measurement time on unnecessary background areas, increasing the measurement speed to up to 100 kHz and the signal-to-noise ratio by several orders of magnitude in comparison to classical raster scanning.
The Femtonics 3D-RTMC provides quasi-instantaneous and simultaneous Real-Time Motion Correction on your in vivo recordings, ensuring immaculate recordings every time. This real-time solution eliminates artifacts caused by heartbeat, breathing or physical activity at over 100 kHz speed allowing calcium and voltage imaging from spines, dendrites, axons and somata with high resolution.
With the implementation of 3D random-access line scanning, we can now correct motion along each axis. Moreover, our approach offers the significant advantage of enabling Z directional (axial) correction just as fast, that surpasses the currently available XY plane cross-correlation-based solutions.
The high-speed arbitrary raster scan mode of the two-photon microscopy based FEMTO3D Atlas provides a scanning speed of 40 fps at 510 x 510 pixels and 500 x 500 µm2, which is faster than most resonant scanning two-photon microscopes, their highest speed being around 30 fps.
Thanks to the AO technology, the scanning plane can be arbitrarily chosen while the speed is being maintained: the plane being scanned with a high speed can be perpendicular to the axis of the objective, or lying at an arbitrary angle in X and/or Y.
Using the 3D random-access excitation method of the two-photon microscopy based FEMTO3D Atlas Dichro, scanning methods of the Femtonics AO technology are available with flexible parameters for photostimulation. Depending on the selected scanning pattern, users can stimulate sparsely distributed individual cells or dendritic processes in a large volume with high precision. By rapidly switching between the two laser lines, activity can be recorded near-simultaneously with photostimulation.
The FEMTO3D ATLAS PLUG & PLAY microscope is a turnkey multiphoton solution: following a smooth delivery to the laboratory, it is ready to operate within an hour. The system can be easily moved within and between laboratories, adapting to your ever-changing needs. While compact in size, the microscope is equipped with the latest 3D acousto-optic (AO) technology for ultra-fast in vivo 3D imaging and 3D photostimulation.
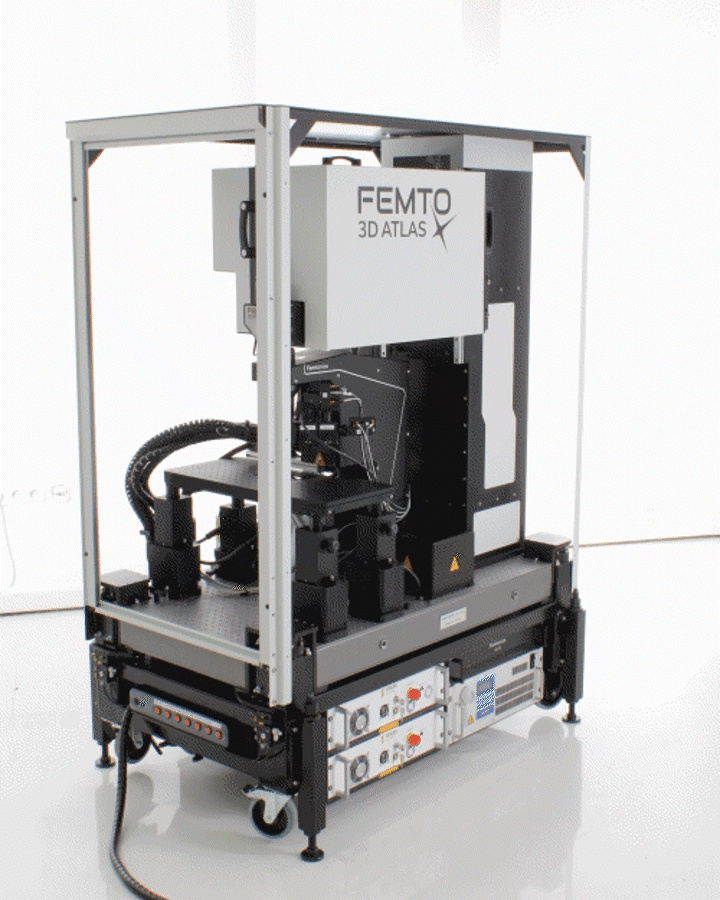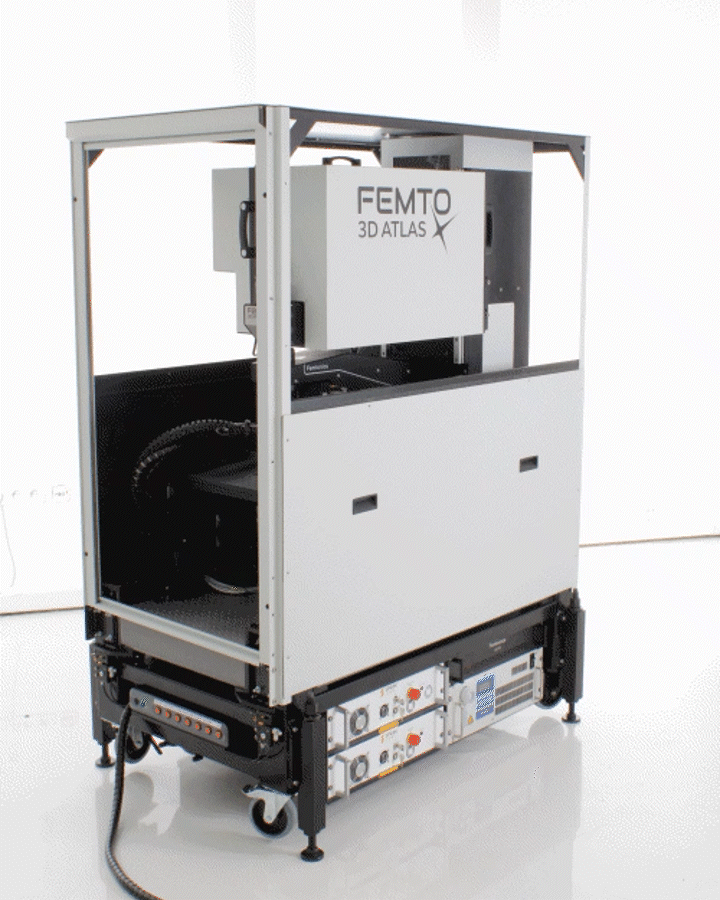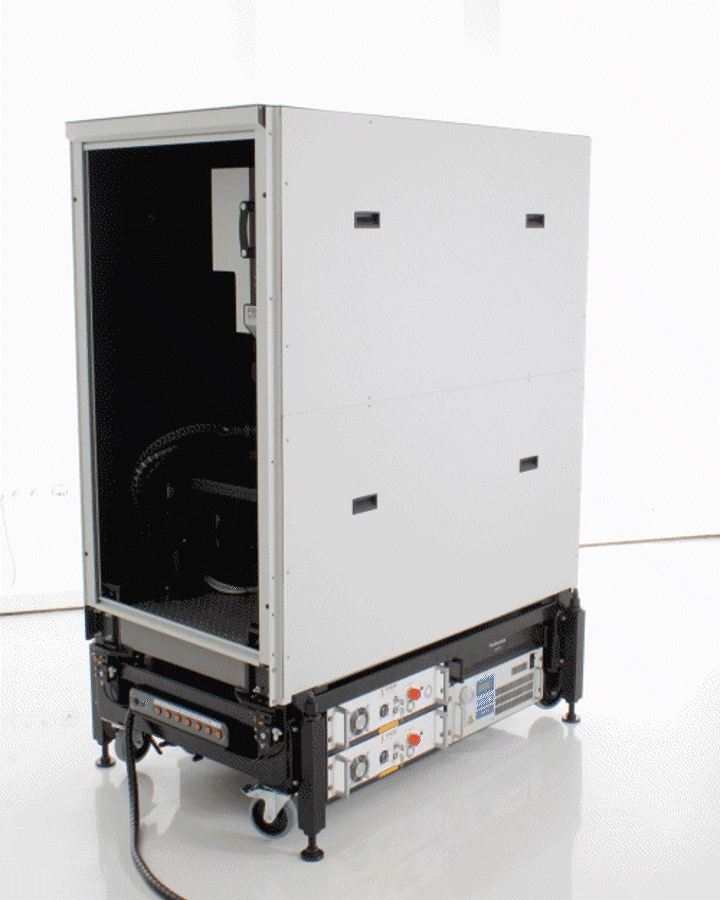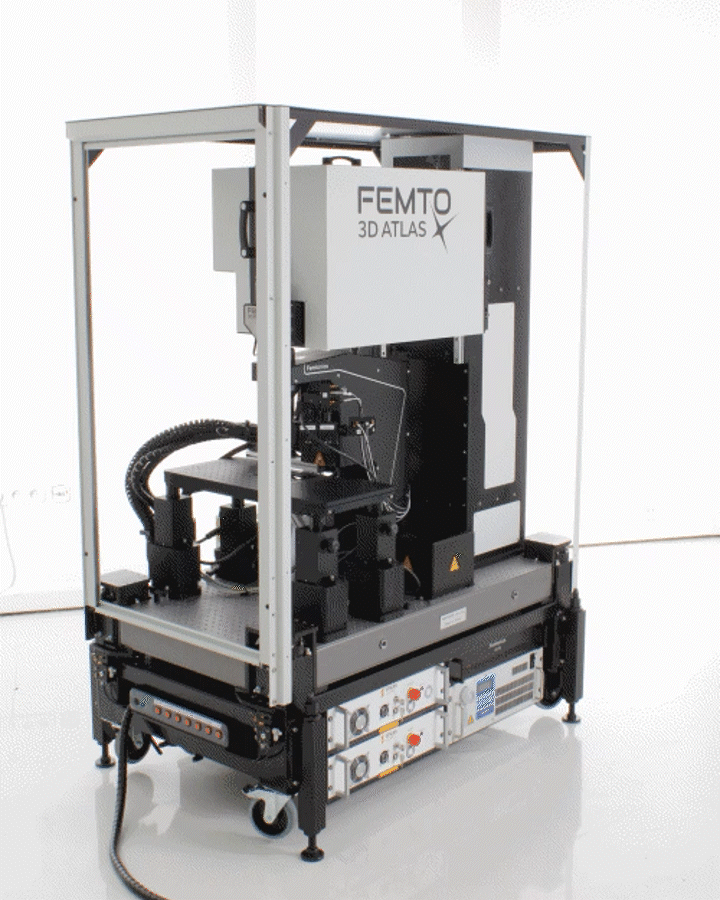
The novel features of the FEMTO3D Atlas microscope, augmenting the advantages of two-photon microscopy, are the electrically tunable acousto-optic deflectors (AODs) responsible for X, Y, and Z focusing (acousto-optic or AO technology). These deflectors do not contain scanning mirrors or any other slowly moving mechanical components, so the positioning of the focal spot is fast, flexible, stable, and independent of the traveling distance. This positioning freedom results in an extremely high scanning speed, up to 100 kHz at any 3D location in a cubic millimeter volume under the objective.
We are proud to have installed our state-of-the-art multiphoton microscopes in prestigious laboratories from all around the world: our users are excellent scientists in their fields. Studies made possible by our systems are being published in high-impact journals. In dedicated Demo Rooms, you can even become acquainted with these systems in their natural habitat. Browse the locations of these labs below, and do not hesitate to contact us if you would like a demonstration organized!
We would like to call your attention to our CENTER OF EXCELLENCE program as well. Besides functioning as Demo Rooms themselves, laboratories and institutes participating in this program will benefit from our constant technological and scientific support and our latest high-tech developments. If you are interested in starting such a fruitful partnership, please, contact our team – we are excited to hear from you.
Budapest, Hungary
 Group Leader: Dr. Balázs Rózsa
Group Leader: Dr. Balázs Rózsa
Two-Photon Imaging Center
Institute of Experimental Medicine
Eötvös Loránd Research Network
Basel, Switzerland

Group Leader: Dr. Botond Roska
Central Visual Circuits & Human Retinal Circuit Groups
Institute of Molecular and Clinical Ophthalmology Basel
Jena, Germany
 Knut Holthoff
Knut Holthoff
Hans Berger Department of Neurology
Jena University Hospital
Genova, Italy

Prof. Antonio Uccelli; Prof. Fabio Benfenati
San Martino Center for Synaptic Neuroscience and Technology (IIT)
Ospedale Policlinico San Martino Genova
Praha, Czech Republik

Institute of Experimental Medicine, Czech Academy of Sciences
Munich, Germany
 Jochen Herms
Jochen Herms
Ludwig Maximilian University of Munich
Washington, USA

Group Leader: Mark Wagner
Neocortex-Cerebellum Circuitry Unit
NIH, Washington D.C.
New York, USA

Attila Losonczy; Christopher Makinson
Department of Neuroscience Mortimer B. Zuckerman
Mind Brain Behavior Institute
Columbia University
Cambridge, USA
 Mriganka Sur
Mriganka Sur
Department of Brain and Cognitive Sciences Massachusetts Institute of Technology
Boston, USA
 Dong Kong
Dong Kong
Harvard University – Boston Children’s Hospital
New Haven, USA
 Tristan Geiller
Tristan Geiller
Yale University
Boston, USA
 Steve Ramirez
Steve Ramirez
Boston University, Center for Neural Systems
Québec, Canada
 Stuart Trenholm
Stuart Trenholm
Life Sciences Complex (McIntyre)
Bejing, China
![]() Prof. Bing Liu
Prof. Bing Liu
Institute of Automation, Chinese Academy of Sciences
 Prof. Yan Gewen
Prof. Yan Gewen
Institute of Zoology, Chinese Academy of Sciences
References
Large-Scale 3D Two-Photon Imaging of Molecularly Identified CA1 Interneuron Dynamics in Behaving Mice. Tristan Geiller, Bert Vancura, Satoshi Terada, Eirini Troullinou, Spyridon Chavlis, Grigorios Tsagkatakis, Panagiotis Tsakalides, Katalin Ócsai, Panayiota Poirazi, Balázs J. Rózsa, Attila Losonczy, Neuron (2020)
Restoring light sensitivity using tunable near-infrared sensors. Dasha Nelidova, Rei K. Morikawa, Cameron S. Cowan, Zoltan Raics, David Goldblum, Hendrik P. N. Scholl, Tamas Szikra, Arnold Szabo, Daniel Hillier, Botond Roska, Science (2020)
CLARITY analysis of the Cl/pH sensor expression in the brain of transgenic mice. Artem V.Diuba, Dmitry V.Samigullin, Attila Kaszas, Francesca Zonfrillo, Anton Malkov, Elena Petukhova, Antonio Casini, Daniele Arosio, Monique Esclapez, Cornelius T. Gross, Piotr Bregestovski, Neuroscience (2019)
Functional Synaptic Architecture of Callosal Inputs in Mouse Primary Visual Cortex. Kuo-Sheng Lee, Kaeli Vandemark, Dávid Mezey, Nicole Shultz, David Fitzpatrick, Neuron (2019)
Fast 3D Imaging of Spine, Dendritic, and Neuronal Assemblies in Behaving Animals. Gergely Szalay, Linda Judak, Gergely Katona, Katalin Ocsai, Gabor Juhasz, Mate Veress, Zoltan Szadai, Andras Feher, Tamas Tompa, Balazs Chiovini, Pal Maak, Balazs Rozsa, Neuron (2016)
Accurate spike estimation from noisy calcium signals for ultrafast three-dimensional imaging of large neuronal populations in vivo. Thomas Deneux, Attila Kaszas, Gergely Szalay, Gergely Katona, Tamas Lakner, Amiram Grinvald, Balazs Rozsa & Ivo Vanzetta, Nature Communications (2016)
Single-cell-initiated monosynaptic tracing reveals layer-specific cortical network modules. Wertz A, Trenholm S, Yonehara K, Hillier D, Raics Z, Leinweber M, Szalay G, Ghanem A, Keller G, Rozsa B, Conzelmann KK, Roska B, Science (2015)
Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Gergely Katona, Gergely Szalay, Pál Maak, Attila Kaszas, Mate Veress, Daniel Hillier, Balazs Chiovini, E Sylvester Vizi, Botond Roska & Balazs Rozsa, Nature Methods (2012)
Localized Neuron Stimulation with Organic Electrochemical Transistors on Delaminating Depth Probes. Williamson A, Ferro M, Leleux P, Ismailova E, Kaszas A, Doublet T, Quilichini P, Rivnay J, Rozsa B, Katona G, Bernard C, Malliaras GG., Advanced Materials (2015)
Dendritic spikes induce ripples in parvalbumin interneurons during hippocampal sharp waves. B Chiovini, G F Turi, G Katona, A Kaszas, D Palfi, P Maak, G Szalay, M F Szabo, Z Szadai, Sz Kali and B Rozsa, Neuron (2014)
Sensitization of neonatal rat lumbar motoneuron by the inflammatory pain mediator bradykinin. Mouloud Bouhadfane, Attila Kaszas, Balazs Razsa, Ronald M Harris-Warrick, Laurent Vinay, Frederic Brocard, eLife (2015)
Unitary GABAergic volume transmission from individual interneurons to astrocytes in the cerebral cortex. Rozsa M, Baka J, Borde S, Rozsa B, Katona G, Tamas G, Brain Struct Funct. (2017)
The team of Prof. Losonczy has been the first ever to create an awe-inspiring 3D inventory of interneuron activity. In their research published in Neuron, (Geiller et al., 2020) the group precisely identified the type of every single interneuron in a large neural network in the brain of behaving mice: they described their anatomical properties using immunohistochemistry, and simultaneously followed their calcium activity in real time in 3D with a high spatiotemporal resolution for the first time ever using FEMTO3D Atlas multiphoton microscope. This way, it was possible to study these cells during such key events for learning and memory formation, as sharp-wave ripples.
